Protein degradation targeting chimeric body as well as preparation method and application thereof
A protein degradation and chimera technology, applied in the fields of drug combination, organic chemistry, anti-tumor drugs, etc., can solve problems such as the advent of protein degradation targeted chimeras
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0051] One embodiment of the present invention further provides a preparation method of the above protein degradation targeting chimera, and the preparation method includes the following steps S11-S14.
[0052] S11. Compound 1 is provided; wherein, compound 1 contains a heterocycle and at least two primary or secondary amine groups, and the heterocycle contains at least one N atom;
[0053] In one of the embodiments, the above compound 1 contains two secondary amine groups.
[0054] In one of the embodiments, the above-mentioned compound 1 contains a primary amino group and a secondary amino group.
[0055] In one embodiment, the aforementioned heterocycle is an aliphatic heterocycle.
[0056] In one of the embodiments, the above-mentioned heterocycle is a five-membered ring or a six-membered ring
[0057] In one embodiment, the above-mentioned compound 1 contains a heterocycle, and the heterocycle is a five-membered ring or a six-membered ring. Further, in one of the embodim...
Embodiment 1
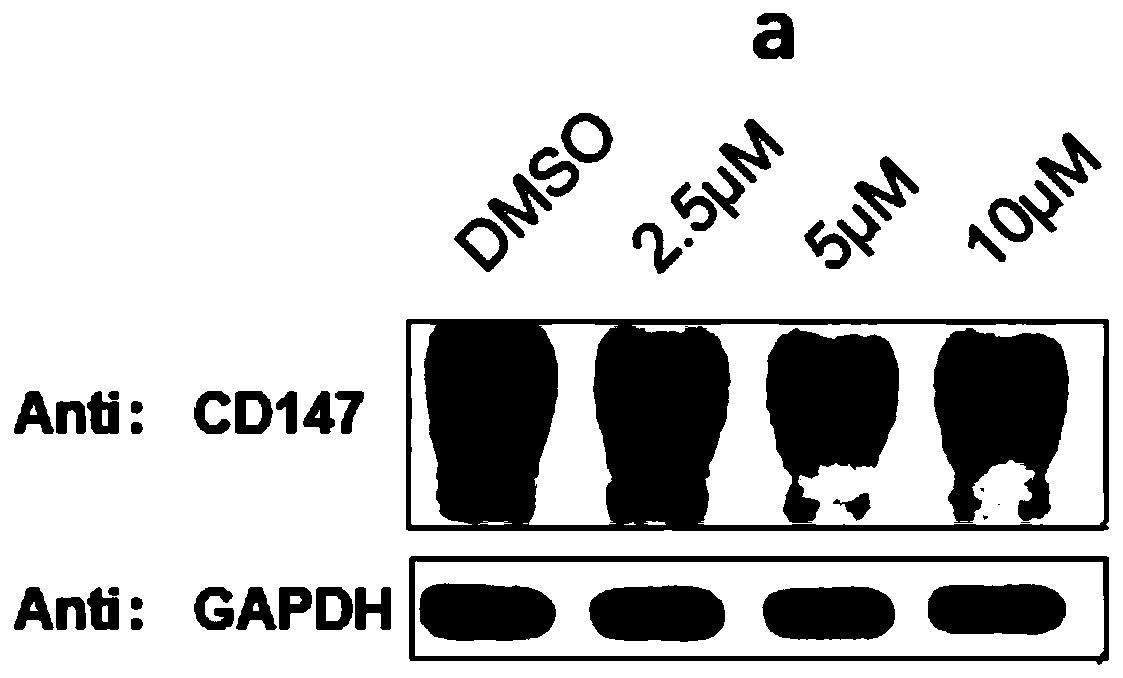
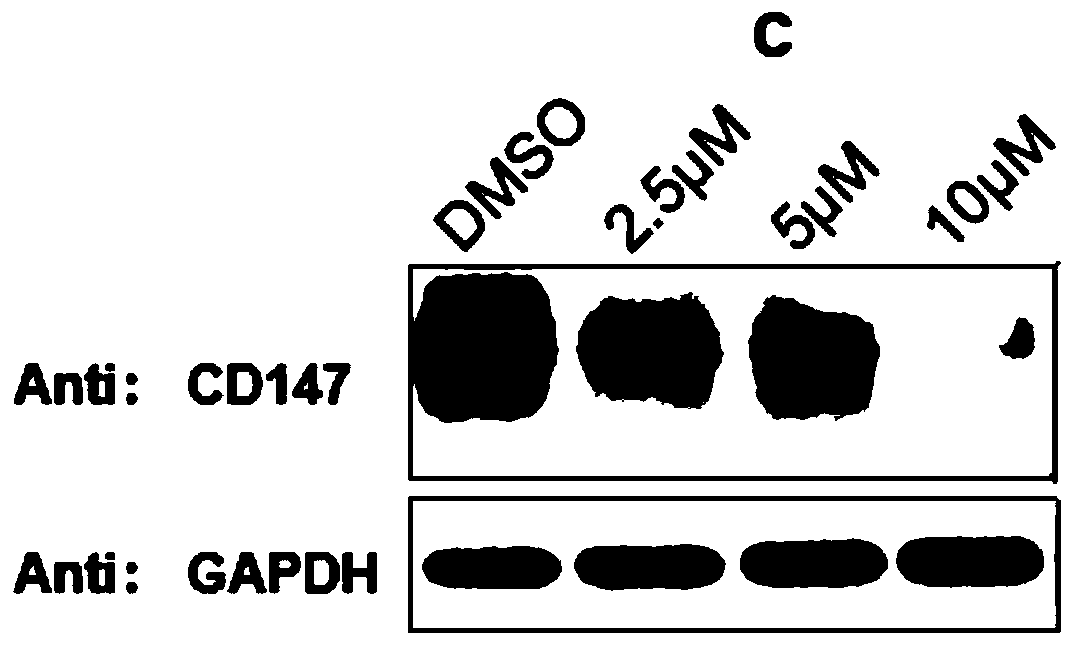
[0124] The structural formula of protein degradation targeting chimera (c) is as follows:
[0125]
[0126] The synthesis steps are as follows:
[0127] (1) Weigh 2-(2,6-dioxo-piperidin-3-yl)-4-fluoro-isoindole-1,3-dione (55mg, 0.2mmol) and 4-Boc -Aminopiperidine (44mg, 0.22mmol), added into a reactor equipped with 2mL NMP, stirred and dissolved, then added 66uL N,N-diisopropylethylamine (DIEA) to the reactor, and heated to The reaction was carried out at 100°C for 4 hours. After cooling, the solvent was spin-dried to obtain a crude product. The crude product was subjected to column chromatography to obtain 78 mg of an intermediate with a yield of 86%. The structure of the intermediate is shown below.
[0128]
[0129] The characterization results of the intermediate are as follows:
[0130] 1 H NMR (500MHz, DMSO) δ: 11.10(s, 1H), 7.71-7.65(m, 1H), 7.33(d, J=7.9Hz, 2H), 6.92(d, J=7.7Hz, 1H), 5.09 (dd,J=12.8,5.5Hz,1H),3.68-3.60(m,2H),3.51-3.40(m,1H),2.98-2.83(m,3H),2...
Embodiment 2
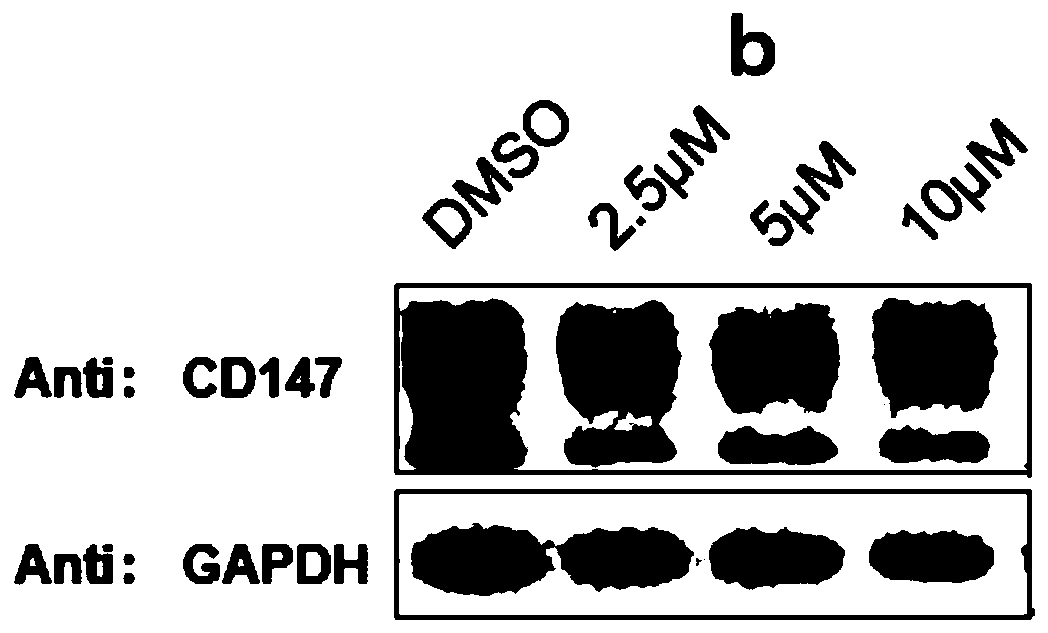
[0136] The structural formula of the protein degradation targeting chimera (b) is as follows:
[0137]
[0138] The synthesis steps are basically the same as in Example 1, except that the 4-Boc-aminopiperidine in the step (1) of Example 1 is replaced with 1-Boc-4-aminopiperidine, and the structure of the obtained intermediate is as follows Show.
[0139]
[0140] The characterization results of the intermediate are as follows.
[0141] 1 H NMR (500MHz, DMSO) δ: 11.11(s, 1H), 7.60(dd, J=8.4, 7.2Hz, 1H), 7.22(d, J=8.7Hz, 1H), 7.06(d, J=7.0Hz ,1H),6.27(d,J=8.4Hz,1H),5.06(dd,J=12.8,5.4Hz,1H),3.97-3.85(m,2H),3.79-3.72(m,1H),3.02- 2.81(m,3H),2.62-2.46(m,4H),2.06-1.99(m,1H),1.95-1.88(m,2H),1.41(s,9H).
[0142] HRMS(ESI)m / z:calcd for C 23 h 28 N 4 NaO 6 (M+Na) + 479.1901,found 479.1904.
[0143] The characterization results of the protein degradation targeting chimera (b) are as follows.
[0144] 1 H NMR (500MHz, DMSO) δ: 8.31(s, 1H), 7.57-7.49(m, 1H), 7.24-7.20(m, 1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com