Modified T cell and preparation method and application thereof
A cell and cell surface technology, applied in the fields of gene editing and tumor immunotherapy, can solve the problems of large tumor heterogeneity, complex tumor microenvironment, and difficult infiltration of CAR-T cells
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0212] Example 1. Effects of CAR-T cells with different CAR structures
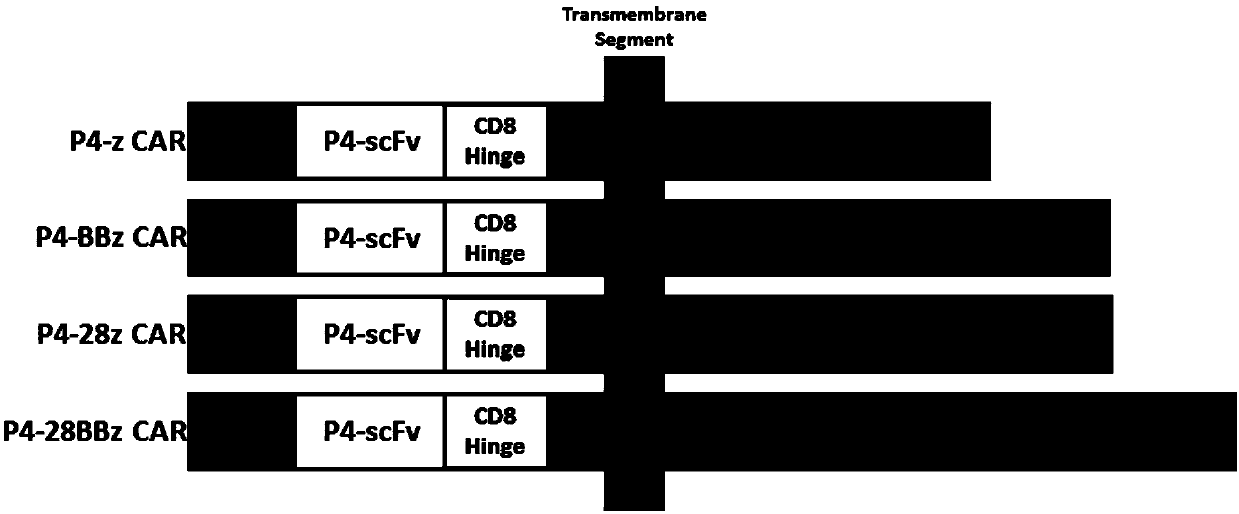
[0213] Four structures of CAR (P4-z, P4-BBz, P4-28z and P4-28BBz were designed, the structures are as follows figure 1 Shown, wherein P4 is anti-mesothelin scFv), and prepare CAR-T cells.
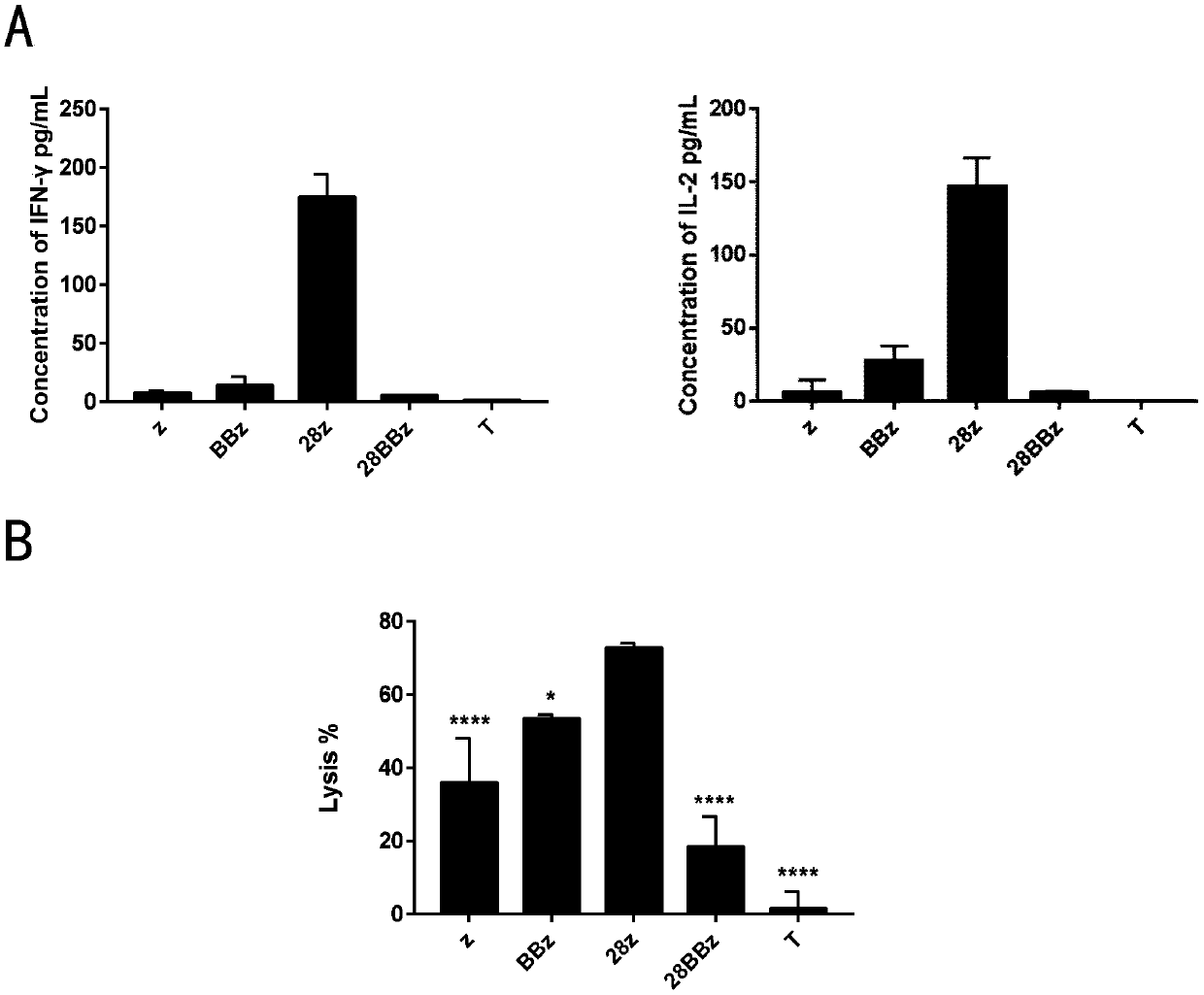
[0214] The resulting CAR-T cells were co-cultured with H226 cells for 20 h at an effector cell:target cell ratio of 1:1, and the release of IFN-γ and IL-2 was measured.
[0215] The resulting CAR-T cells were co-cultured with luciferase-expressing H226 cells (H226-luci) at a ratio of 2:1 effector cells:target cells for 3 days, and the target cells were calculated by measuring the luciferase activity of the remaining tumor cells. Percentage of cell lysis.
[0216] Such as figure 2 As shown, compared with P4-z, P4-BBz and P4-28BBz, P4-28z showed higher release of IFN-γ and IL-2 ( figure 2 A), and higher specific cell lysis ( figure 2 B, * means P<0.05, **** means P<0.0001).
[0217] The following experiments all...
Embodiment 2
[0218] Example 2. The effect of gene editing of inhibitory receptors in CAR-T cells on tumor killing
[0219] This example studies the effect of inhibitory receptors TIGIT, BTLA, CD160, 2B4 and CD200R in CAR-T cells being knocked out through gene editing on tumor killing.
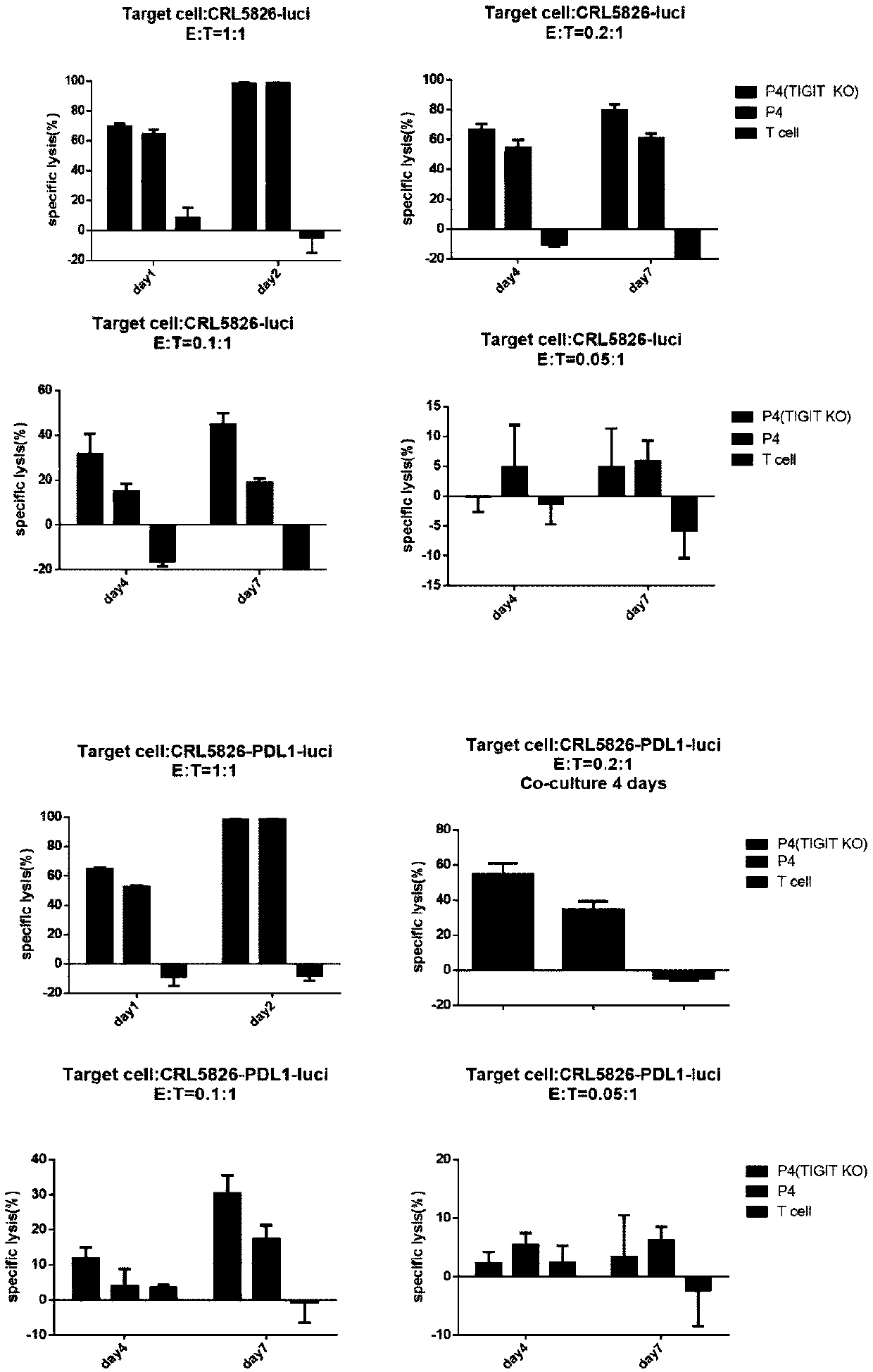
[0220] The TIGIT knockout group showed a killing effect not lower than that of the control CAR-T cells in the short-term (24h, 48h) killing of target cells with a high-efficiency target ratio (1:1) of CRL5826-luci and CRL5826-PDL1-luci, It shows a stronger killing advantage than control CAR-T cells in the killing of low-efficiency targets for a long time. ( image 3 )
[0221] The TIGIT knockout group showed a killing effect not lower than that of the control CAR-T cells in the short-term (24h, 48h) killing of the target cells OVCAR3-luci and HCT116-luci with a high-efficiency target ratio (1"1). The effect target ratio showed a stronger killing advantage than the control CAR-T cells in the long-term kil...
Embodiment 3
[0230] Example 3. The effect of gene editing of T cell exhaustion-related transcription factors in CAR-T cells on tumor killing
[0231] This example studies the effect of T cell exhaustion-related transcription factors BATF, GATA3, IRF4, and RARA in CAR-T cells being knocked out through gene editing on tumor killing.
[0232] The BATF knockout group showed a killing effect not lower than that of the control CAR-T cells in the short-term (24h, 48h) killing of target cells with high-efficiency target ratio (1:1) of CRL5826-luci and CRL5826-PDL1-luci, It has a very obvious killing advantage over control CAR-T cells in the killing of low-efficiency targets and long-term killing. ( Figure 13 )
[0233] The BATF knockout group showed a killing effect not lower than that of the control CAR-T cells in the short-term (24h, 48h) killing of the target cells OVCAR3-luci and HCT116-luci with a high-efficiency target ratio (1:1). Compared with the control CAR-T cells, the effect target...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com