Humanized antihuman CTLA4 monoclonal antibody and preparation method and application thereof
A monoclonal antibody, humanized technology, applied in the field of tumor immunotherapy and molecular immunology, can solve problems such as side effects, achieve high affinity, achieve tumor immunotherapy, and high specificity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0085] Example 1 Humanization of mouse-derived anti-human CTLA4 antibody
[0086] 1) The sequence of the mouse-derived anti-human CTLA4 antibody 42B11G12D3 (the CDR region is underlined) (see, for example, SEQ ID NO: 1-2)
[0087]
[0088]
[0089] 2) Construction of anti-human CTLA4 antibody CDR grafted plasmid
[0090] Select the IMGT human V gene (F+ORF+in-frameP) database, select the human Germline antibody sequence with the highest homology as the humanized receiving vector according to the comparison, and transfer the three heavy chain complementarity determining regions HCDR1 and HCDR2 in the mouse antibody and HCDR3, the three light chain complementarity determining regions LCDR1, LCDR2 and LCDR3 were transferred to the corresponding positions, and the post-translational modification sites (PTM) were analyzed, as shown in Table 1. Sequence analysis revealed that two sites, W33 and M63, are hotspots for post-translational oxidative modification (see eg, SEQ ID NO...
Embodiment 2
[0124] Example 2: Recombinant Production of Humanized Antibody
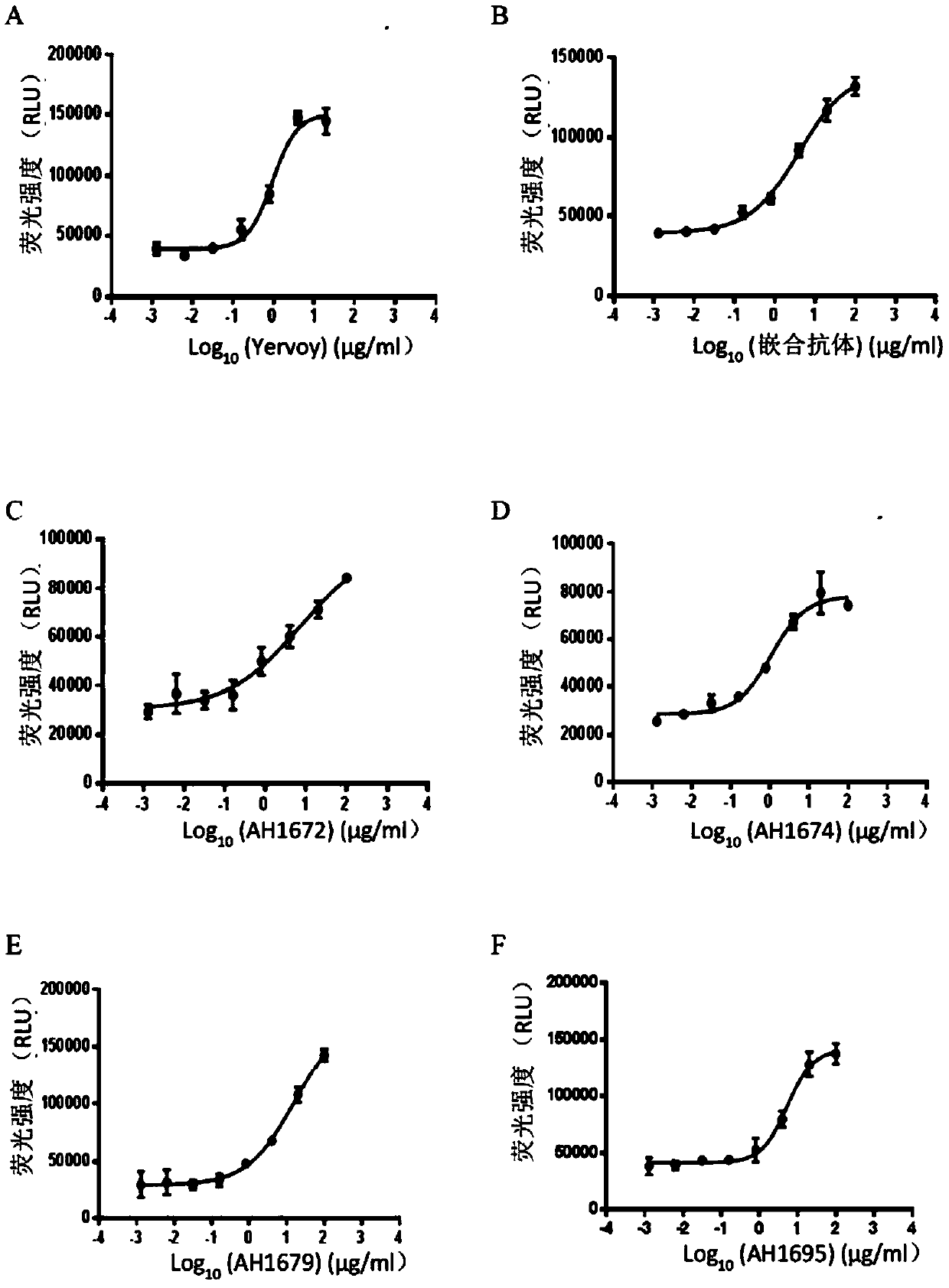
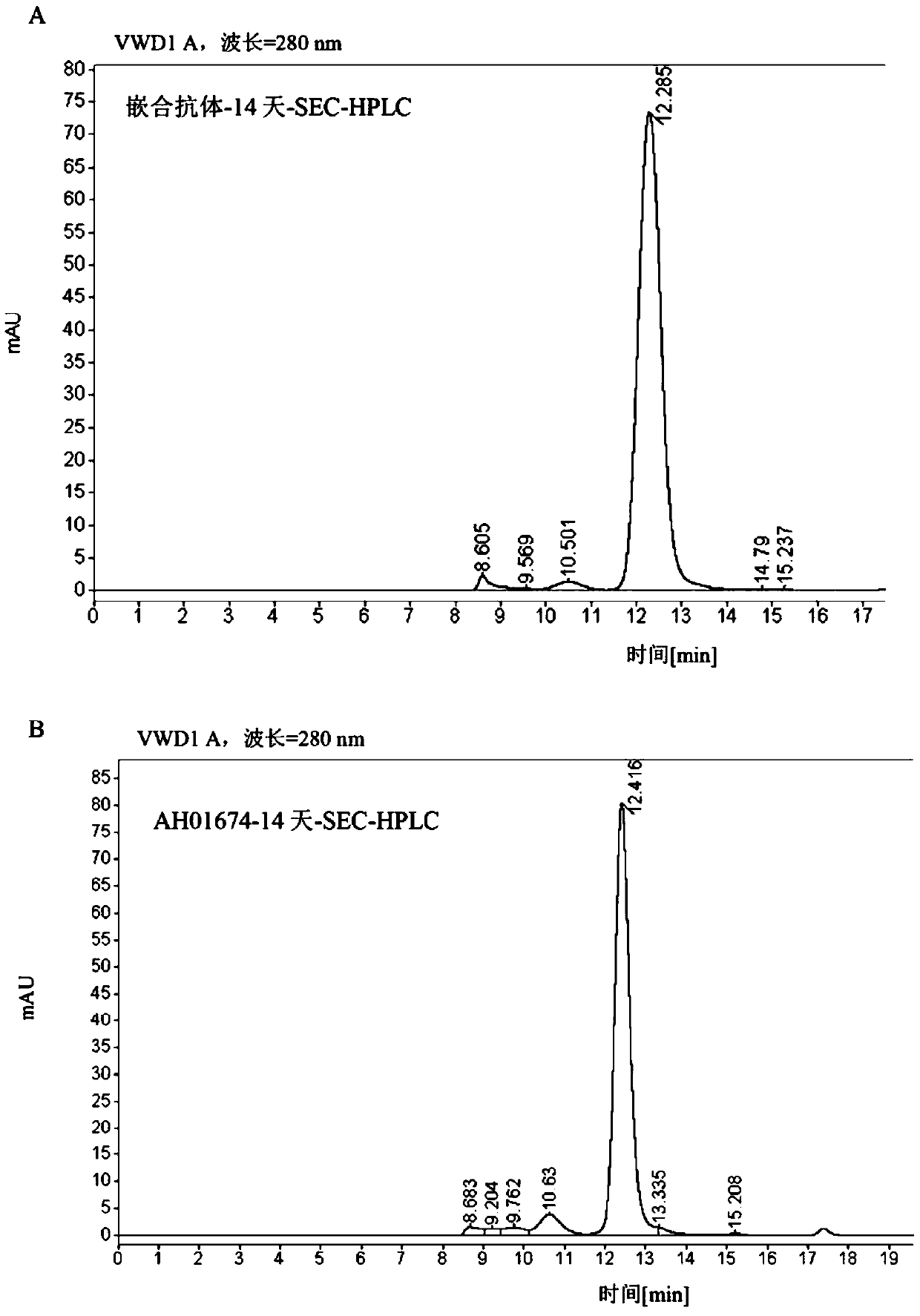
[0125] The selected antibody VH and VL sequences were codon-optimized, connected to the 5' end of the secretory signal peptide, connected to the human antibody IgG1 heavy chain, kappa light chain constant region sequences, respectively cloned into the pTT5 expression vector to prepare the Human antibody DNA sequence expressed and secreted in mammalian cells. The plasmid was co-transfected into HEK293-6E suspension culture cells with PEI for transient expression. During transfection, the cell density was maintained at 1 × 10 6 cells / mL with a PEI:DNA ratio of 3:1. Cells at 37 °C 5% CO 2 Shake culture at 105 rpm in the incubator. 24 hours after transfection, 0.5% Trypton N-1 was added. After 5 days, the cell culture supernatant was collected, and the antibody was purified using protein-A agarose gel, quantified and identified for purity (Table 3).
[0126] Table 3: Recombinant production of humanized antibodie...
Embodiment 3
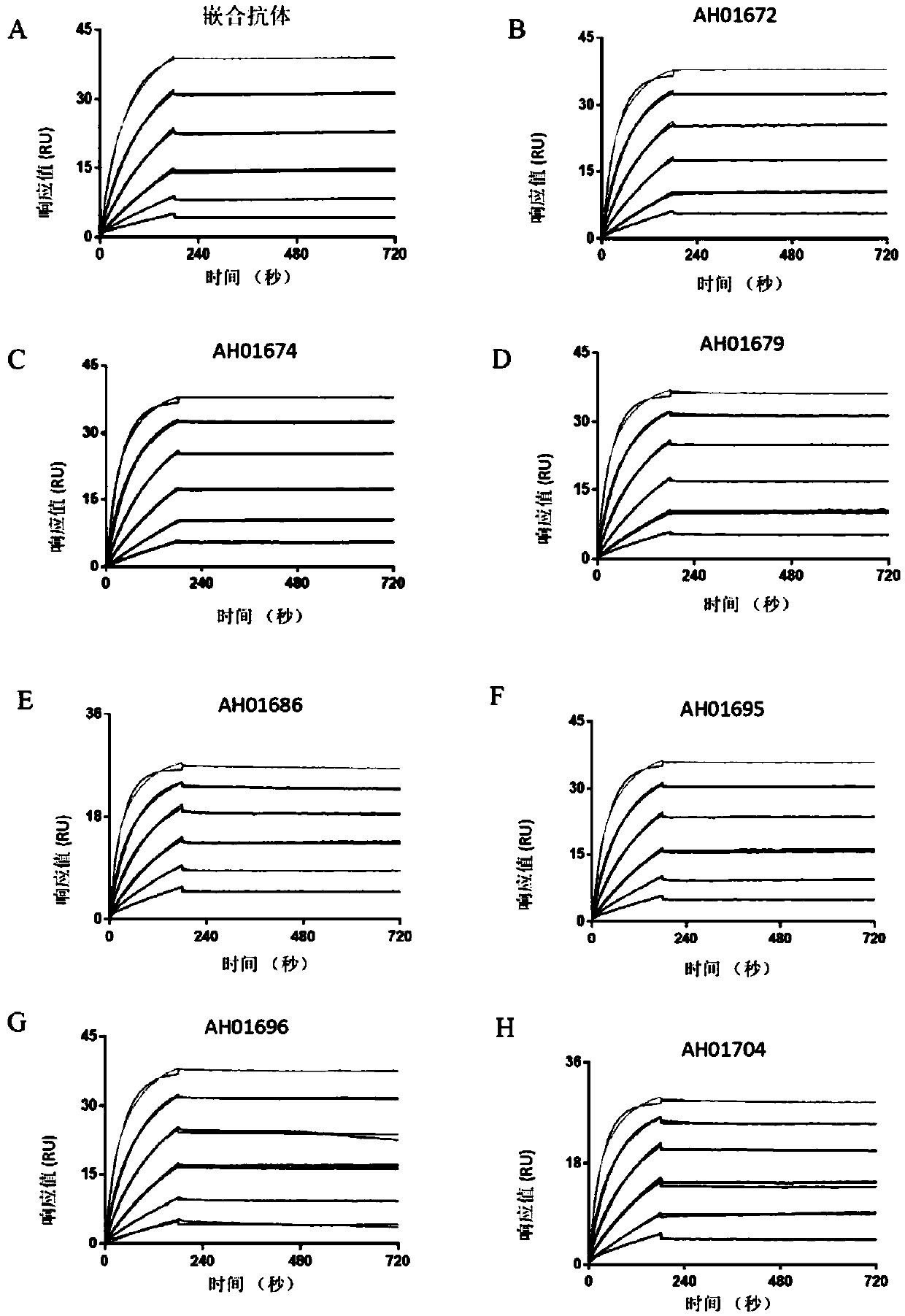
[0129] Example 3: Affinity Determination of Humanized Monoclonal Antibodies
[0130] Equilibrate the surface of the chip with HBS-EP buffer at a flow rate of 10 μl / min for 5 minutes, then inject a 1:1 mixture of "NHS+EDC" at a flow rate of 10 μl / min for 7 minutes to activate the chip, dilute in 10 mM sodium acetate buffer The capture antibody (Goat anti-mouse IgG) was injected at a flow rate of 10 μl / min for about 7 minutes for coupling, and finally ethanolamine was injected at a flow rate of 10 μl / min for 7 minutes for surface blocking.
[0131] Use HBS-EP buffer as the sample for three pre-circulations to balance the chip to stabilize the baseline, and inject the antibody diluted in HBS-EP buffer at a flow rate of 10 μl / min for 0-5 minutes (control the binding of antibody and antigen by adjusting the capture time Signal at ~100RU), buffer equilibrated for 1 min. Inject low-concentration antigen 0.33nM CTLA4-Fc at a flow rate of 30 μl / min for 5 minutes to bind the antigen to...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com