Application of CA125 to curative effect evaluation of primary liver cancer stereotactic radiotherapy
A technology for CA125 and primary liver cancer, which is applied in the direction of measuring devices, instruments, scientific instruments, etc., can solve the problems that primary liver cancer does not have good diagnostic performance and prognosis evaluation performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] Example 1. Evaluation of diagnostic sensitivity of tumor markers in patients with liver cancer
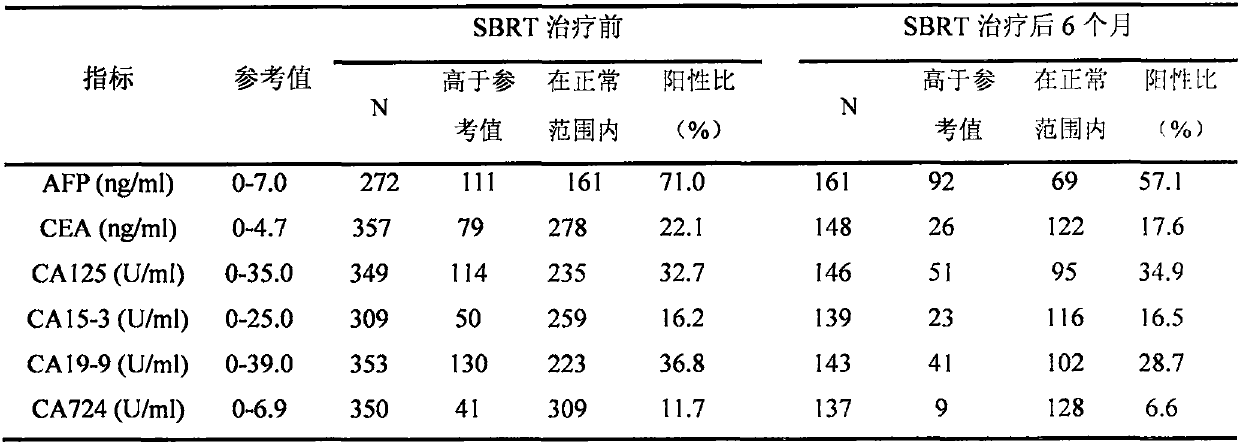
[0018] Collected the medical records of patients with liver cancer treated with SBRT for the first time in tertiary hospitals from July 2012 to December 2018, a total of 598 cases (the same patient was admitted to the hospital multiple times, counted as 1 case), excluding SBRT combined with chemotherapy, targeted therapy, etc. There were 202 patients with other treatment methods, and a total of 396 patients with liver cancer who only received SBRT were obtained. At the end of December 2018, 396 patients were followed up for the last time, and the SPSS database was completed. According to the concentration of tumor markers AFP, CEA, CA125, CA15-3, CA19-9 and CA724 in the serum of patients before and 6 months after SBRT treatment, evaluate the effect of each tumor marker before and 6 months after SBRT treatment in patients with liver cancer sensitivity.
[0019] In the popula...
Embodiment 2
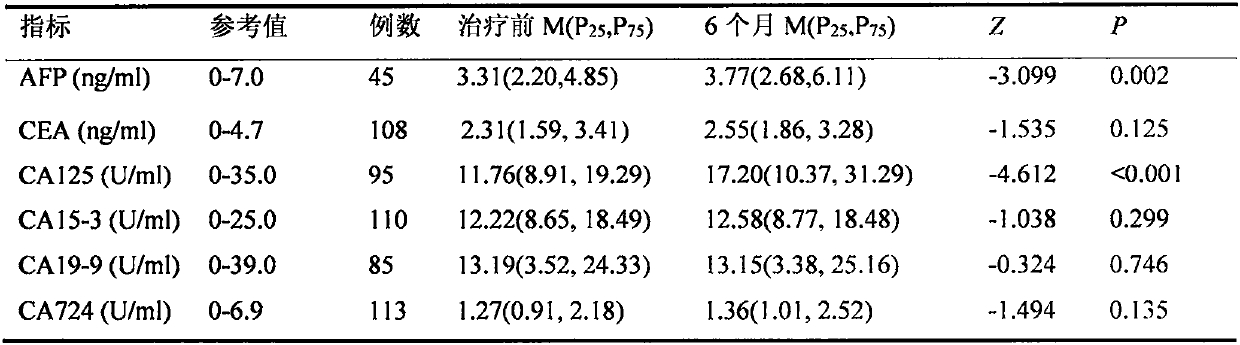
[0023] Example 2. For the population whose tumor markers were within the normal range before SBRT treatment, which tumor markers were screened for differences before and after SBRT treatment
[0024] In Example 1, only alpha-fetoprotein AFP has a high sensitivity in diagnosing liver cancer. Since more than half of liver cancer patients have normal levels of tumor markers before and after SBRT treatment, can another method be used for liver cancer patients with negative tumor markers? To evaluate the efficacy of SBRT treatment is our research interest.
[0025]In order to answer the above questions, we screened out the population of 396 liver cancer patients whose tumor marker concentrations were within the normal range before SBRT treatment and whose corresponding tumor marker concentrations were detected 6 months after treatment. The paired rank sum test was used to analyze the changes of AFP, CEA, CA125, CA15-3, CA19-9, and CA724 in this population before and 6 months after ...
Embodiment 3
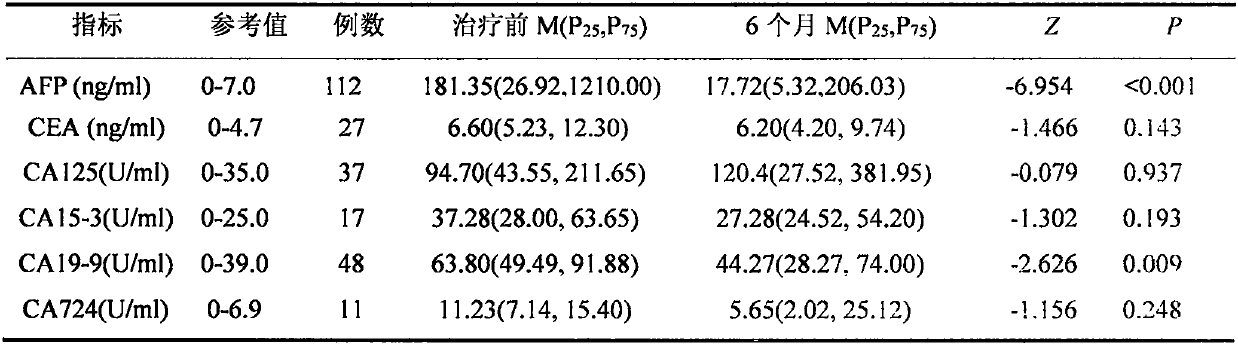
[0030] Example 3. Evaluation of the influence of the change ratio of CA125 at 6 months after SBRT treatment on the prognosis of 1 to 5 years
[0031] In Example 2, we found that in patients with primary liver cancer whose tumor markers were within the normal reference range before SBRT treatment, only AFP and CA125 had significant differences before and after SBRT treatment. In this example, we calculated the ratio of changes in AFP and CA125 after 6 months of SBRT compared with before treatment in patients with primary liver cancer who were followed up for 1 year, 2 years, 3 years and 5 years [(6 months Concentration-concentration before treatment) / concentration before treatment] in the survival group and death group, the results showed that AFP had no change before and after treatment; while the change ratio of CA1256 months was compared to 1 year, 2 years, 3 years and 5 years The follow-up prognosis was affected, and there were significant differences between the survival g...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Sensitivity | aaaaa | aaaaa |
| Sensitivity | aaaaa | aaaaa |
| Sensitivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com