Application of ATF3 in preparation of PD-L1/PD-1 monoclonal antibody tumor immunotherapy drug
A PD-L1, anti-tumor immunity technology, applied in the field of biology, PD-L1/PD-1 monoclonal antibody tumor immunity, can solve problems such as unclear mechanism of action
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
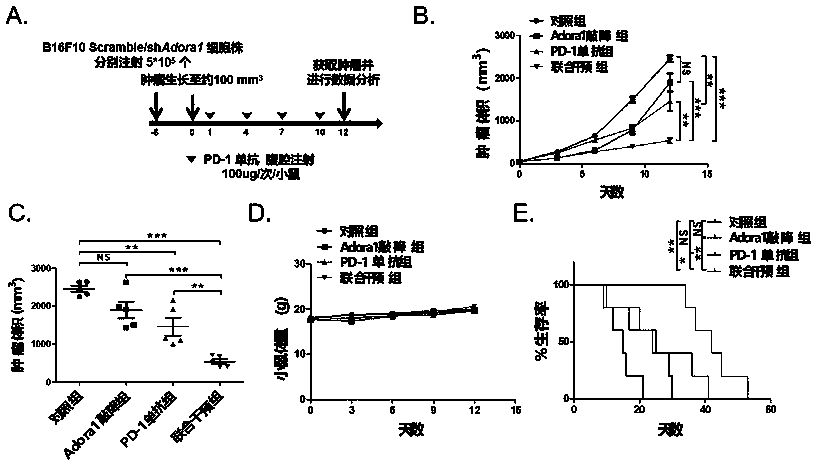
[0038] 1. In vivo study on the proliferation of adenosine receptor ADORA1 combined with PD-1 monoclonal antibody against transplanted tumor (melanoma) in mice.
[0039] 1.1 Group settings:
[0040] Scramble+IgG2a (control group)
[0041] shAdora1+IgG2a (Adora1 knockdown group)
[0042] Scramble+PD-1mAb (PD-1 mAb treatment group)
[0043] shAdora1+PD-1mAb (joint intervention group)
[0044] 1.2 Experimental process, see figure 1 A:
[0045] About 6 days before the experiment: 6-8 weeks old C57BL / 6 mice were subcutaneously injected with B16F10 melanoma shAdora1 knockdown cell line 5*10 in the right dorsal wing. 5 20 mice each and the same number of Scramble cell lines (control group).
[0046] Day 0 of the experiment: record the body weight and tumor volume of the mice, when the tumor volume grows to about 100mm 3 Beginning at the beginning of time, mice inoculated with shAdora1 knockdown cell line and Scramble cell line were injected intraperitoneally with PD-1mAb 100ug / ...
Embodiment 2
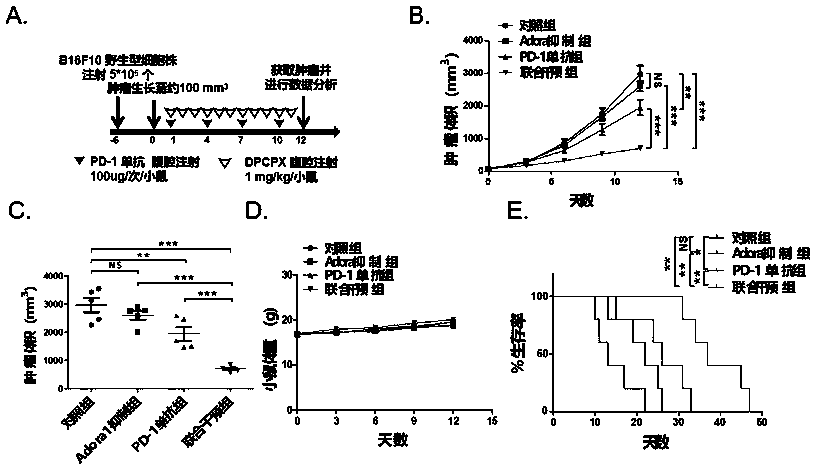
[0062] 1. The in vivo study of the specific inhibitor DPCPX targeting and inhibiting the proliferation of adenosine receptor ADORA1 combined with PD-1 monoclonal antibody transplanted tumors (melanoma, non-small cell lung cancer) in mice.
[0063] 1.1 Group settings:
[0064] Vehicle+IgG2a (control group)
[0065] DPCPX+IgG2a (Adora1 inhibition group)
[0066] Vehicle+PD-1mAb (PD-1 mAb treatment group)
[0067] DPCPX+PD-1mAb (joint intervention group)
[0068] 1.2 Experimental process, see image 3 A. 4A:
[0069] About 6 days before the experiment: 6-8 weeks C57BL / 6 mice were subcutaneously injected with B16F10 melanoma 5*10 in the right dorsal wing 5 Individual or LLC non-small cell lung cancer 1*10 6 wild-type cell lines, 40 mice each.
[0070] Day 0 of the experiment: record the body weight and tumor volume of the mice, when the tumor volume grows to about 100mm 3 At the beginning of time, the inoculated mice were divided into four groups, respectively, DPCPX 1mg / K...
Embodiment 3
[0094] 1. In vivo study of gene knockdown of transcription factor ATF3 combined with adenosine receptor ADORA1 specific inhibitor DPCPX against proliferation of mouse xenograft tumors (melanoma, non-small cell lung cancer).
[0095] 1.1 Group settings:
[0096] Scramble+vehicle (control group)
[0097] Scramble+DPCPX (Adora1 inhibition group)
[0098] ShAtf3+vehicle (Atf3 knockdown group)
[0099] ShAtf3+DPCPX (joint intervention group)
[0100] 1.2 Experimental process, see Figure 5 A. 6A:
[0101] About 6-8 days before the experiment: 6-8 weeks old C57BL / 6 mice were subcutaneously injected with B16F10 melanoma shAtf3 knockdown cell line 5*10 in the right dorsal wing respectively. 5 Individual or LLC non-small cell lung cancer shATF3 knockdown cell line 1*10 6 , and the same number of Scramble cell lines (control group), 20 mice each.
[0102] Day 0 of the experiment: record the body weight and tumor volume of the mice, when the tumor volume grows to about 100mm 3 Be...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com