Homogeneous immunoassay method and equipment based on proximity strike effect and graphene oxide quenching acridinium ester chemiluminescence
A technique for chemical and immunological analysis of cyanidinium esters, which is applied in the fields of chemiluminescence/bioluminescence, analysis of materials through chemical reactions, and analysis of materials. There are problems such as stability problems, to achieve the effect of shortened turnaround time, lower requirements, and fewer modules
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
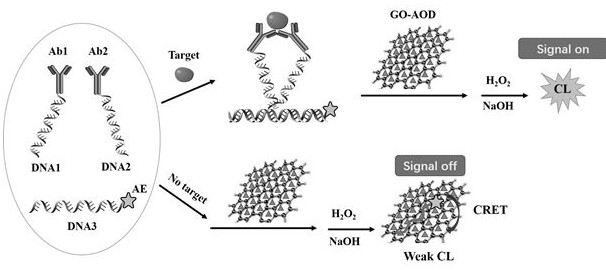
[0073] Embodiment 1: in combination with figure 1 , illustrating the detection of troponin I in whole blood by a homogeneous immunoassay based on graphene oxide-antioxidant quenched acridinium ester chemiluminescence
[0074] 1. Configure detection reagents: mix DNA1-antibody 1 conjugate, DNA2-antibody 2 conjugate, AE-modified DNA3, GO-AOD (AOD is vitamin C), so that their final concentrations are 1-20nM, 1-20 nM, 0.05-0.2 μM and 20 μg / ml (optimal conditions are 10 nM, 10 nM, 0.15 μM and 20 μg / ml).
[0075] 2. Mix 50 μL of calibration solutions of different concentrations or whole blood samples containing troponin I with 200 μL of detection solution, place them in the detection well of the rotor, start the incubation, incubate at 37°C for 5-10 minutes, and the incubation is complete. The detection hole is turned to the detection position, facing the light source module.
[0076] 3. Through the control of the circuit board module and the host computer software, start the step...
Embodiment 2
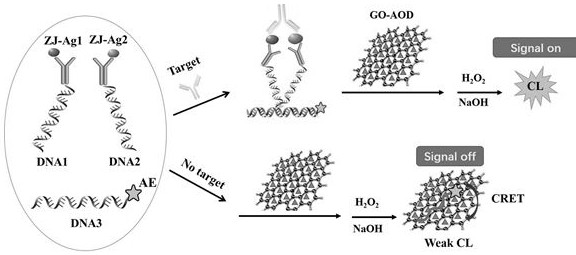
[0083] Embodiment 2: in combination with figure 2 , illustrating the detection of hepatitis B surface antibody (HBsAb) in whole blood by a homogeneous immunoassay based on graphene oxide-antioxidant quenched acridinium ester chemiluminescence
[0084] 1. Configure detection reagents: mix DNA1-scaffold protein 1 conjugate (ZJ-Ag1), DNA2-antibody protein 2 conjugate (ZJ-Ag2), AE-modified DNA3, GO-AOD (AOD is vitamin E) mixed , so that their final concentrations were 1-20nM, 1-20nM, 0.05-0.2μM and 20μg / ml (the optimal conditions were 10nM, 10nM, 0.15μM and 20μg / ml). Scaffolds are project-independent immunoglobulins IgG.
[0085] 2. Mix 50 μL of calibration solutions of different concentrations or whole blood samples containing HBsAb with 200 μL of detection solution, place them in the detection well of the rotor, start incubation, and incubate at 37°C for 5 minutes. After the incubation is completed, the detection well is turned to detection The position is directly opposite t...
Embodiment 3
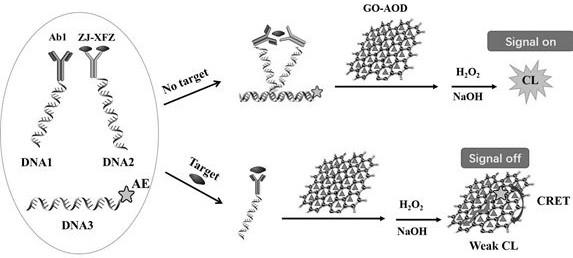
[0091] Embodiment 3: in combination with image 3 , illustrating the detection of free thyroxine (fT4) in whole blood by a homogeneous immunoassay based on graphene oxide-antioxidant quenched acridinium ester chemiluminescence
[0092] 1. Configure the detection reagent: mix DNA1-antibody 1 conjugate, DNA2-scaffold small molecule conjugate (ZJ-XFZ), AE-modified DNA3, GO-AOD (AOD is cannabidiol) to make their final The concentrations are 1-5nM, 5-20nM, 0.05-0.2μM and 20μg / ml (optimum conditions are 1nM, 10nM, 0.1μM and 20μg / ml). Scaffolds are project-independent immunoglobulins IgG.
[0093] 2. Mix 50 μL of calibration solutions of different concentrations or whole blood samples containing fT4 with 200 μL of detection solution, place them in the detection well of the rotor, start incubation, and incubate at 37°C for 5 minutes. After the incubation is completed, the detection well is turned to detection The position is directly opposite to the light source module.
[0094] 3....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com