Methods and compositions for treating cancer
A therapeutic composition and composition technology, applied in the field of cancer treatment and composition, capable of solving problems such as exhausted T cells, decreased lymphocytes in mice, and off-target effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0172] ways to treat cancer
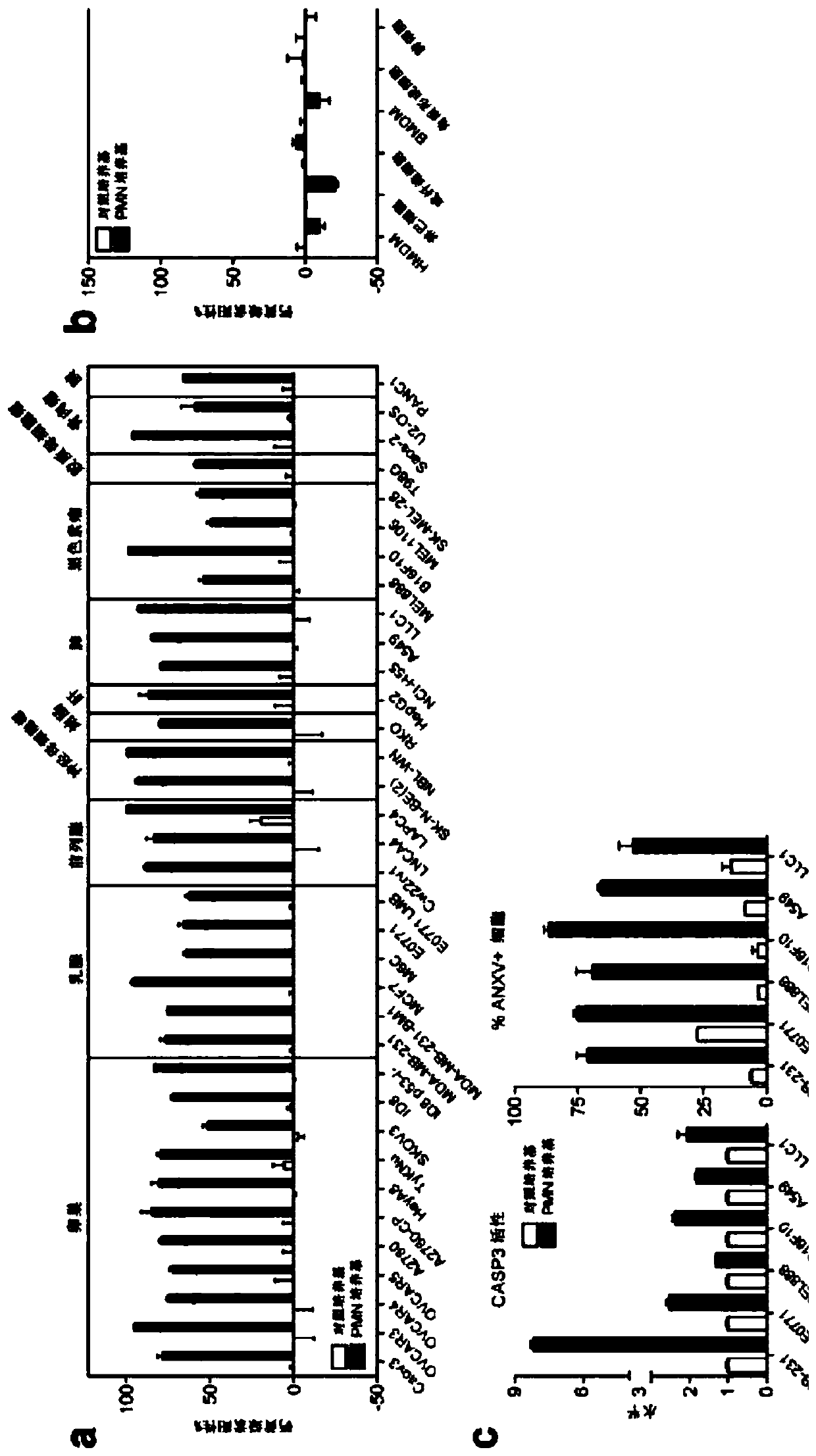
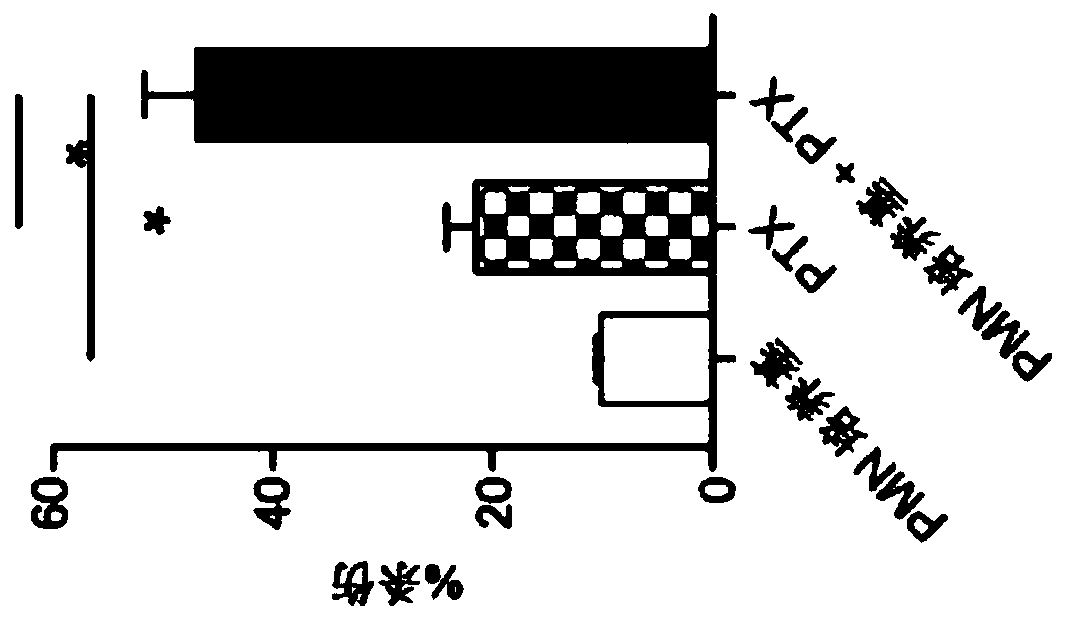
[0173] Human PMNs broadly and selectively kill cancer cells. Human peripheral blood neutrophils (PMN) were isolated from healthy donors and incubated in serum-free DMEM to collect its secreted factors (PMN medium). (A-B) Human or murine cancer cells (A) or healthy cells (b) were incubated with PMN medium or control serum-free DMEM (control medium) for 24 hours. Cell viability was assessed by calcein AM staining. (C) Human or murine cancer cells were treated with PMN medium or control medium for 6 hours. Caspase 3 / 7 activity was detected by luminescence assay and cell surface annexin V staining was assessed by flow cytometry. The results showed that PMN medium induced the death of cancer cells through apoptosis. *, p<0.05, Student's t-test (Figure 1).
[0174] Human neutrophil-adapted medium kills a variety of cancer cells. Human peripheral blood neutrophils were isolated from healthy donors. Cells were seeded in serum-free DMEM, and neutrop...
Embodiment 2
[0185] Degradation of CD95 and Cancer Therapy
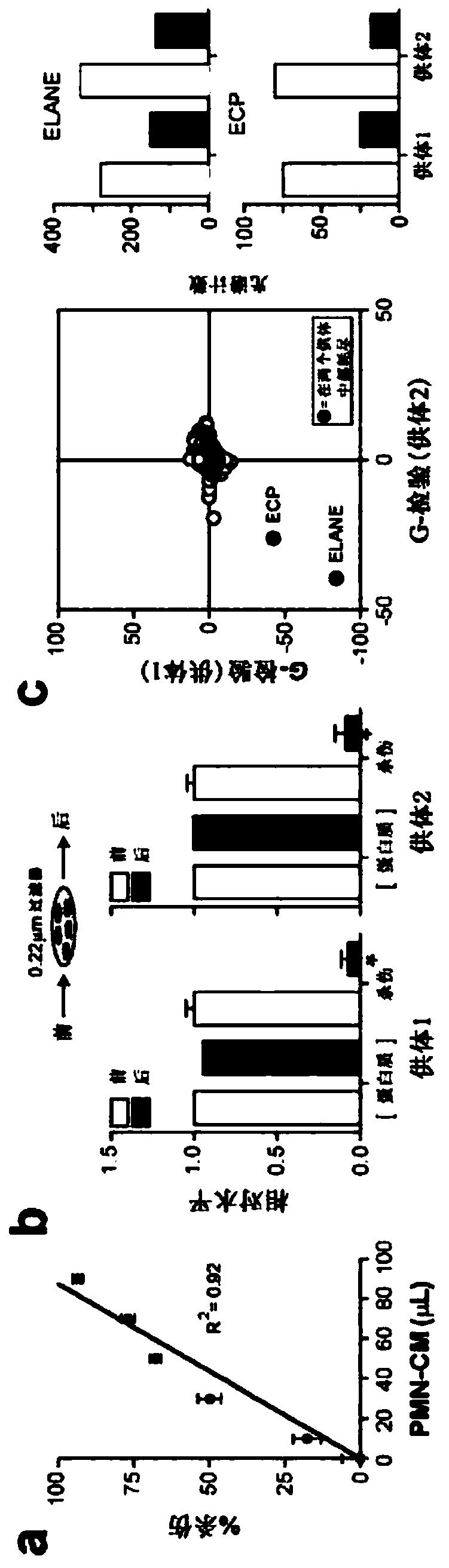
[0186] The catalytic activity of ELANE is required for its selective killing of cancer cells. (Fig. 5A) Purified native ELANE or PMN medium was treated with PMSF (100 nM) or α-1-antitrypsin (A1AT; 42 nM) for 30 min, and the loss of ELANE catalytic activity was confirmed by chromogenic substrate assay. Killing assays were performed by treating MDA-MB-231 cells for 24 h and assessed by calcein AM staining. (FIG. 5B) ELANE activity in PMN medium was measured by chromogenic substrate activity assay, and PMN-killing effect on MDA-MB-231 cells exposed to PMN medium for 4 hours was measured by calcein AM staining. The results showed that the catalytic activity of ELANE in PMN medium was linearly correlated (linearly) with the cancer cell killing ability of PMN medium from nine healthy donors. *, p<0.05, Student's t-test.
[0187] ECP is a type II allosteric activator of ELANE catalytic activity. (FIG. 7A) 1OnM ELANE was incubated wi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com