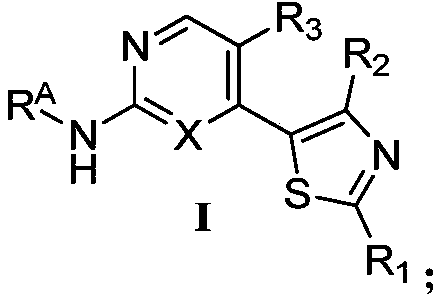
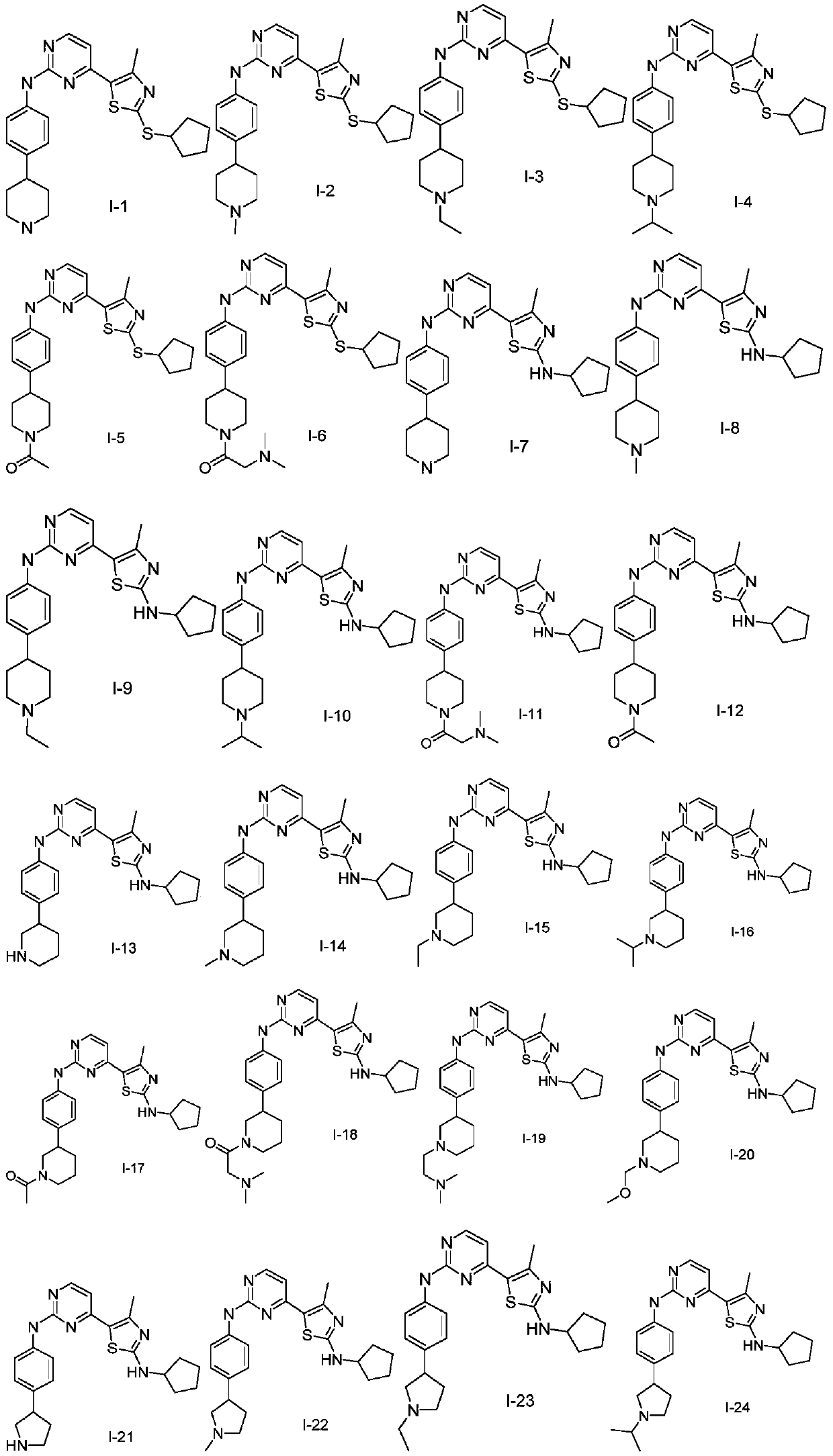
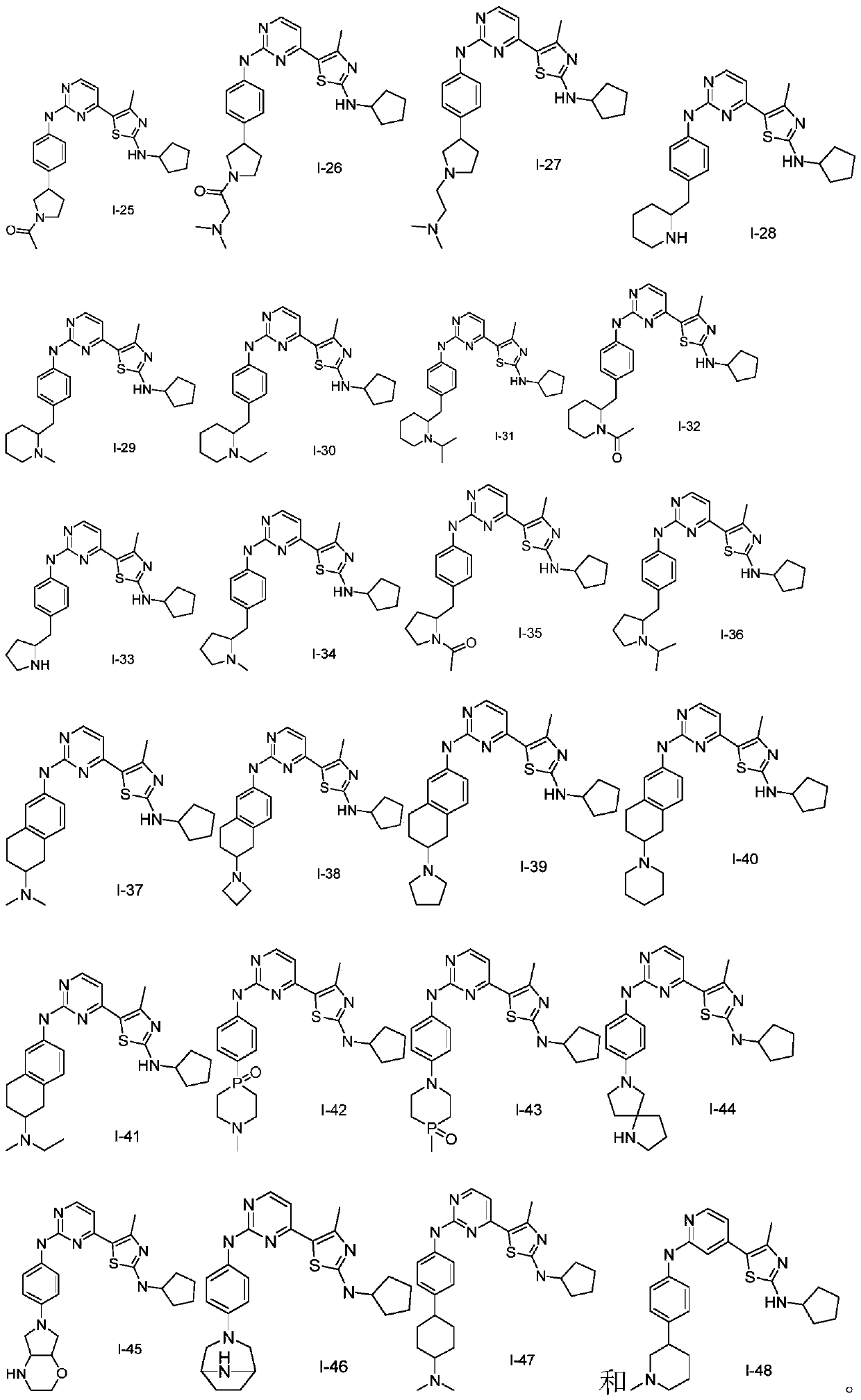
Nitrogen-containing heterocyclic compound as well as preparation method and application thereof
A nitrogen-heterocyclic compound and solvate technology, which is applied in the field of nitrogen-containing heterocyclic compounds, can solve problems such as uncontrolled cell proliferation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 7
[0274]
[0275] first step:
[0276] 5-(2-chloropyrimidin-4-yl)-N-cyclopentyl-4-methyl-thiazol-2-amine (50mg, 0.17mmol), 4-(4-aminophenyl)piperidine-1 - tert-butylcarboxylate (50mg, 0.18mmol), cesium carbonate (100mg), 4,5-bisdiphenylphosphine-9,9-dimethylxanthene (10mg, 0.017mmol), tris(di Benzylideneacetone) dipalladium (10 mg) was added to 1,4-dioxane (3 ml), and the temperature was raised to 110° C. under the protection of argon to react for 12 hours. After cooling to room temperature, filter with diatomaceous earth, and the filtrate is concentrated and subjected to silica gel column chromatography (methanol / dichloromethane 0-5%) to obtain compound 4-[4-[[4-[2-(cyclopentylamino) -4-Methyl-thiazol-5-yl]pyrimidin-2-yl]amino]phenyl]piperidine-1-tert-butylcarboxylate (90mg, 0.168mmol).
[0277] Step two:
[0278] 4-[4-[[4-[2-(Cyclopentylamino)-4-methyl-thiazol-5-yl]pyrimidin-2-yl]amino]phenyl]piperidine-1-tert-butylcarboxy Add ester (90mg, 0.168mmol) to 2M HCl / MeOH solu...
Embodiment 8
[0282]
[0283] 5-(2-chloropyrimidin-4-yl)-N-cyclopentyl-4-methyl-thiazol-2-amine (50mg, 0.17mmol), 4-(1-methylpiperidin-4-yl ) aniline (34mg, 0.18mmol), cesium carbonate (100mg), 4,5-bisdiphenylphosphine-9,9-dimethylxanthene (10mg, 0.017mmol), tris(dibenzylideneacetone) Dipalladium (10 mg) was added into 1,4-dioxane (3 ml), and the temperature was raised to 110° C. under the protection of argon to react for 12 hours. After cooling to room temperature, filter with diatomaceous earth, and the filtrate is concentrated and subjected to silica gel column chromatography (methanol / dichloromethane 0-5%) to obtain the compound N-cyclopentyl-4-methyl-5-[2-[4 -(1-Methyl-4-piperidine)anilino]pyrimidin-4-yl]thiazol-2-amine (20 mg, 0.045 mmol).
[0284] 1 H NMR (400MHz, MeOD) δ8.26(d, J=5.5Hz, 1H), 7.65(d, J=8.5Hz, 2H), 7.20(d, J=8.5Hz, 2H), 6.90(d, J =5.5Hz,1H),4.12–3.92(m,1H),3.38(s,2H),2.90–2.62(m,6H),2.53(s,3H),2.14–1.85(m,7H),1.84– 1.73(m,2H),1.70–1.54(m,5H).
[0285] LC-MS: m...
Embodiment 14
[0287]
[0288] first step:
[0289] 5-(2-aminopyrimidin-4-yl)-N-cyclopentyl-4-methyl-thiazol-2-amine (90mg, 0.33mmol), 3-(4-bromophenyl)piperidine-1 - tert-butylcarboxylate (108mg, 0.32mmol), cesium carbonate (250mg), 4,5-bisdiphenylphosphine-9,9-dimethylxanthene (20mg), tris(dibenzylidene Acetone) dipalladium (20 mg) was added into 1,4-dioxane (6 ml), and the temperature was raised to 110° C. under the protection of argon to react for 12 hours. After cooling to room temperature, filter with diatomaceous earth, and the filtrate is concentrated and subjected to silica gel column chromatography (methanol / dichloromethane 0-5%) to obtain compound 3-[4-[[4-[2-(cyclopentylamino) -4-Methyl-thiazol-5-yl]pyrimidin-2-yl]amino]phenyl]piperidine-1-tert-butylcarboxylate (100mg, 0.187mmol).
[0290] Step two:
[0291] 3-[4-[[4-[2-(Cyclopentylamino)-4-methyl-thiazol-5-yl]pyrimidin-2-yl]amino]phenyl]piperidine-1-tert-butylcarboxy Add ester (100mg, 0.187mmol) to 2M HCl / MeOH solution (4...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com