A kind of olefin thioether staple peptide and its preparation method and application
A technology of olefin thioether and staple peptide, which is applied in the field of medicine, can solve the problems of staple peptide diastereoisomer impurities, limit the promotion and application of staple peptide, and difficult separation and purification, and achieve convenient synthesis and stability in vivo The effect of long resistance and good membrane permeability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
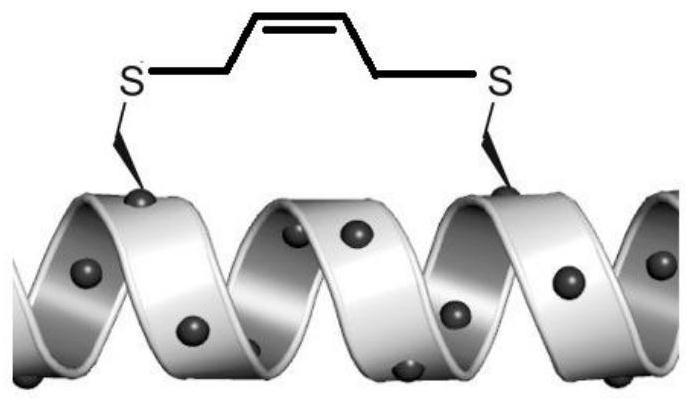
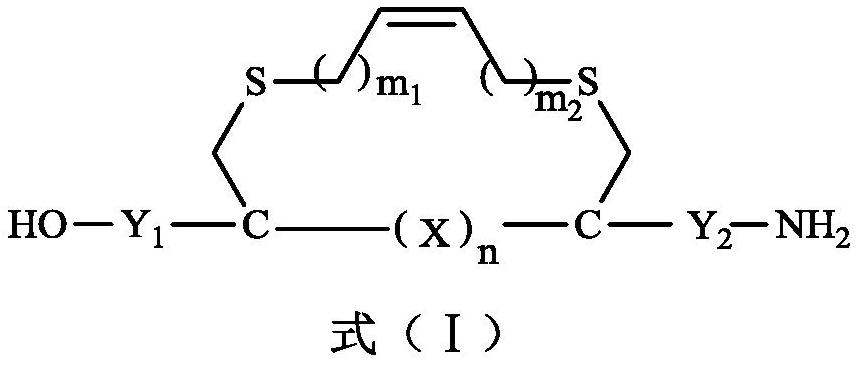
[0032] The methods for the preparation of stapled peptides reported in the existing literature all start from amino acids, first protect them, then cyclize, selectively introduce olefin side chains, and then open the rings, remove the protecting groups, etc., generally need seven or eight steps of reaction to prepare get. Because of its long synthetic route and expensive raw materials, the application of its products is limited. In addition, the linear polypeptide in the early staple peptide is to cut out a functional polypeptide in the protein, and then use the existing amino acids in this polypeptide to select the binding site. In this way, the optional binding site is very popular. Restrictions are not conducive to the formation of the staple bridge.
[0033] Cysteine contains a sulfhydryl functional group, which is often used as a binding site in the synthesis of stapled peptides, but the existing methods all directly bind cysteine in linear polypeptides by chemical m...
Embodiment 1
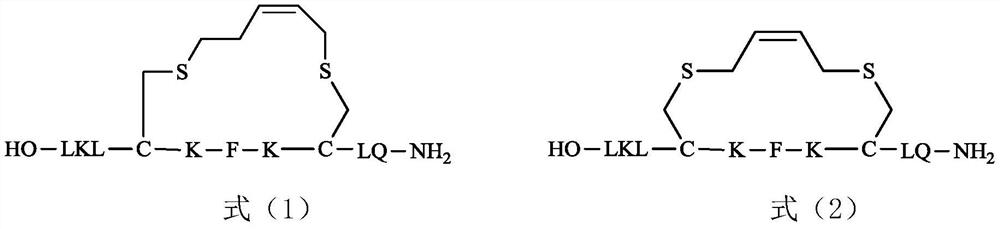
[0045] Example 1: Synthesis of (XL-1)
[0046] (1) unnatural amino acid Fmoc-Cys (C 3 -C 5 Synthesis of -ene)-OH
[0047] Synthesis of Fmoc-Cys(Allyl)-OH
[0048] 2.98g (24.7mmol) of L-cysteine was placed in a 250mL flask, 75mL of ethanol was added, ice bathed, 3.50g (87.5mmol) of sodium hydroxide was dissolved in 45mL of water and added to the reaction system. After stirring at room temperature for 10 minutes, 3.30 g (24.7 mmol) of 3-bromopropene was added, and the stirring reaction was continued for 3 hours. Glacial acetic acid was added until the pH of the reaction system = 5.5, a large amount of white solids were precipitated, filtered and dried in vacuo to obtain 2.50 g of solids. This solid was placed in a 100mL single-necked flask, 50mL of water was added, 1.90g of sodium bicarbonate (22.6mmol) was added to the reaction system in batches, 6.54g of 9-fluorenylmethylsuccinimidyl carbonate (Fmoc-OSu) ( 19.4mmol) was dissolved in 25mL tetrahydrofuran, added into th...
Embodiment 2
[0057] Example 2: Synthesis of (XL-2)
[0058] Carry out by the method for embodiment 1. 0.30 g Wang resin (loading rate 0.63 mmol / g, 0.19 mmol) was synthesized by standard solid-phase peptide synthesis method. After separation and purification, 38 mg of the target stapled peptide (named XL-2) was obtained. HR MS m / z: for [M] + , calculated 1289.641, found 1289.604.
[0059] Using the same preparation process as XL-2, only changing Fmoc-Cys(Butyl)-OH to Fmoc-Cys(Allyl)-OH, the compound of formula (4) was obtained and confirmed to be the compound of formula (4).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com