Method for evaluating CAR-T killing activity in vitro
A technology with killing activity and specificity, applied in the field of cell biology, can solve problems such as inability to meet
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] 1. Experimental materials:
[0048] SKBR3 cells; CD19 virus LV-CD19 (26043-1) (Jikai Gene); infection enhancer polybrene (Jikai Gene); puromycin (vicmed); PE-CD19 flow cytometry antibody (Sanjian Bio); DMEM medium ( Hyclone) (containing 10% FBS);
[0049] Instrumentation: Flow Cytometry (BD); RTCA (Agilent Bio); Microscope (Olympus).
[0050] 2. Experimental method:
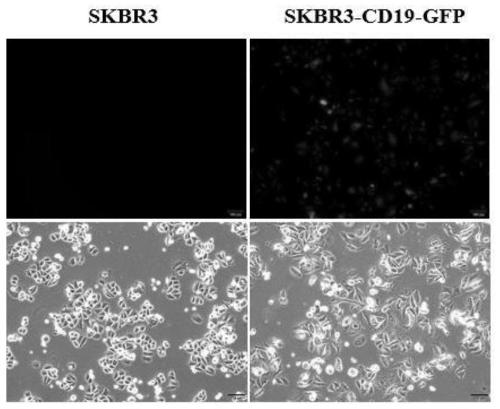
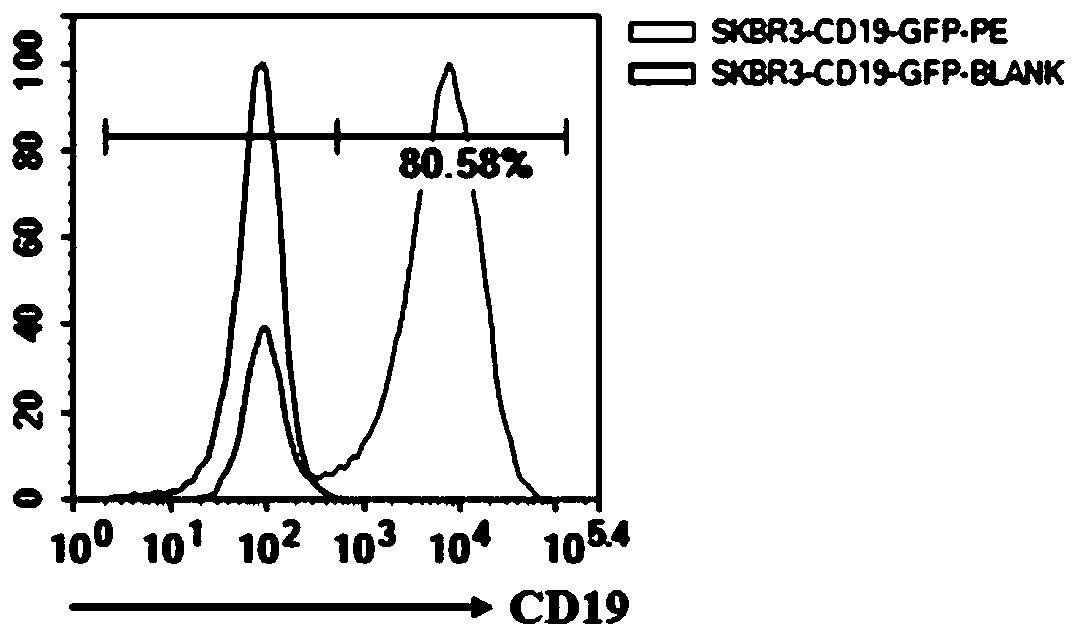
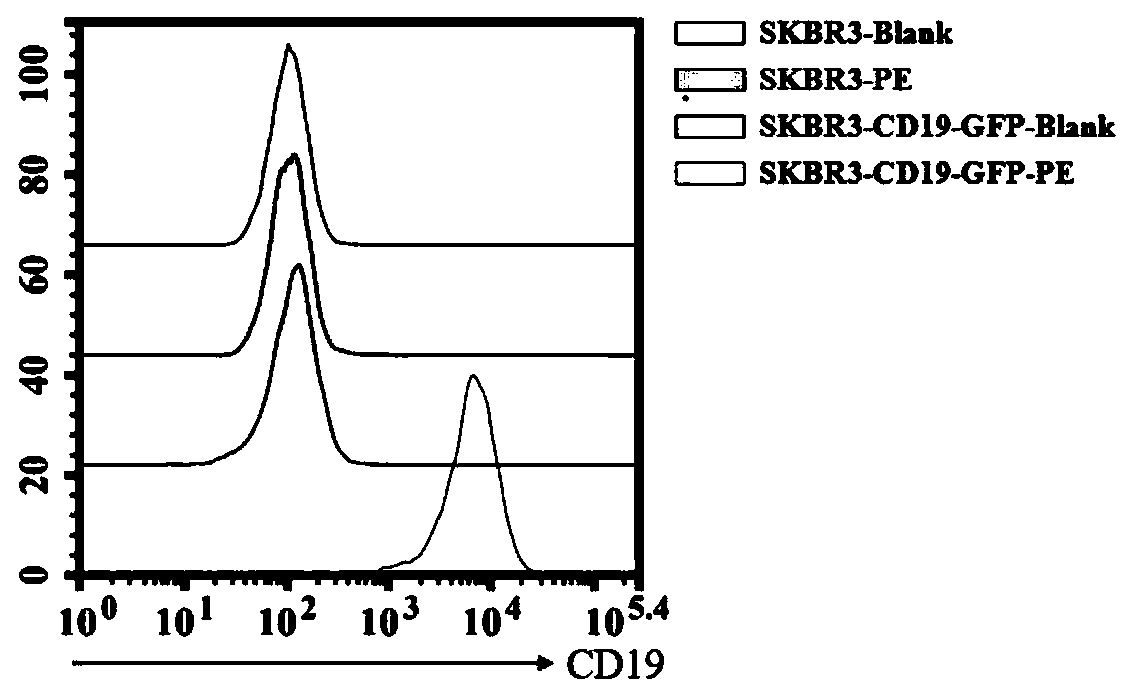
[0051] 1. CD19 virus infected SKBR3 cells.
[0052] The optimal infection conditions (including the amount of inoculated cells, the infection reagent and the time of changing the medium after infection) and the multiplicity of infection (MOI) of the virus to SKBR3 cells were determined by the pre-infection experiment. The specific operation steps are as follows:
[0053] (1) One day before infection, SKBR3 cells in good growth state were prepared with DMEM medium (containing 10% FBS) at a density of 3.75×10 5 cells / ml cell suspension, the cell suspension was 2ml / well (7.5×10 5 Cells / well) were inocul...
Embodiment 2
[0082]1. Experimental materials:
[0083] MDA-MB-231 cells; CD19 virus LV-CD19 (26043-1) (Jikai Gene); infection enhancer polybrene (Jikai Gene); puromycin (vicmed); PE-CD19 flow antibody (Sanjian Biological); DMEM medium (Hyclone) (containing 10% FBS);
[0084] Instrumentation: Flow Cytometry (BD); RTCA (Agilent Bio); Microscope (Olympus).
[0085] 2. Experimental method:
[0086] 1. CD19 virus infected MDA-MB-231 cells.
[0087] The optimal infection conditions (including the amount of inoculated cells, infection reagents, and the time of changing medium after infection) and the multiplicity of infection (MOI) of the virus on MDA-MB-231 cells were determined through the pre-infection experiment. The specific operation steps are as follows:
[0088] (1) One day before infection, the MDA-MB-231 cells in good growth state were prepared with DMEM medium (containing 10% FBS) at a density of 3.75×10 5 cells / ml cell suspension, the cell suspension was 2ml / well (7.5×10 5 Cells...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com