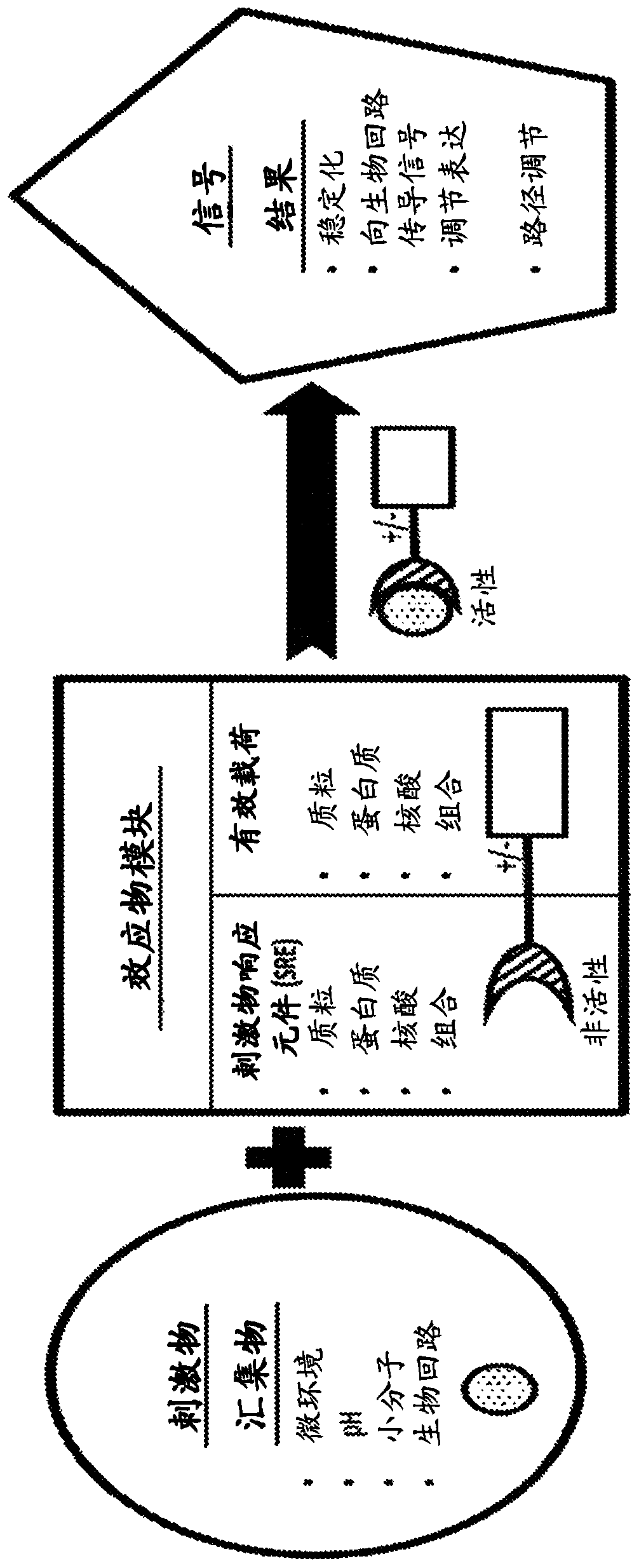
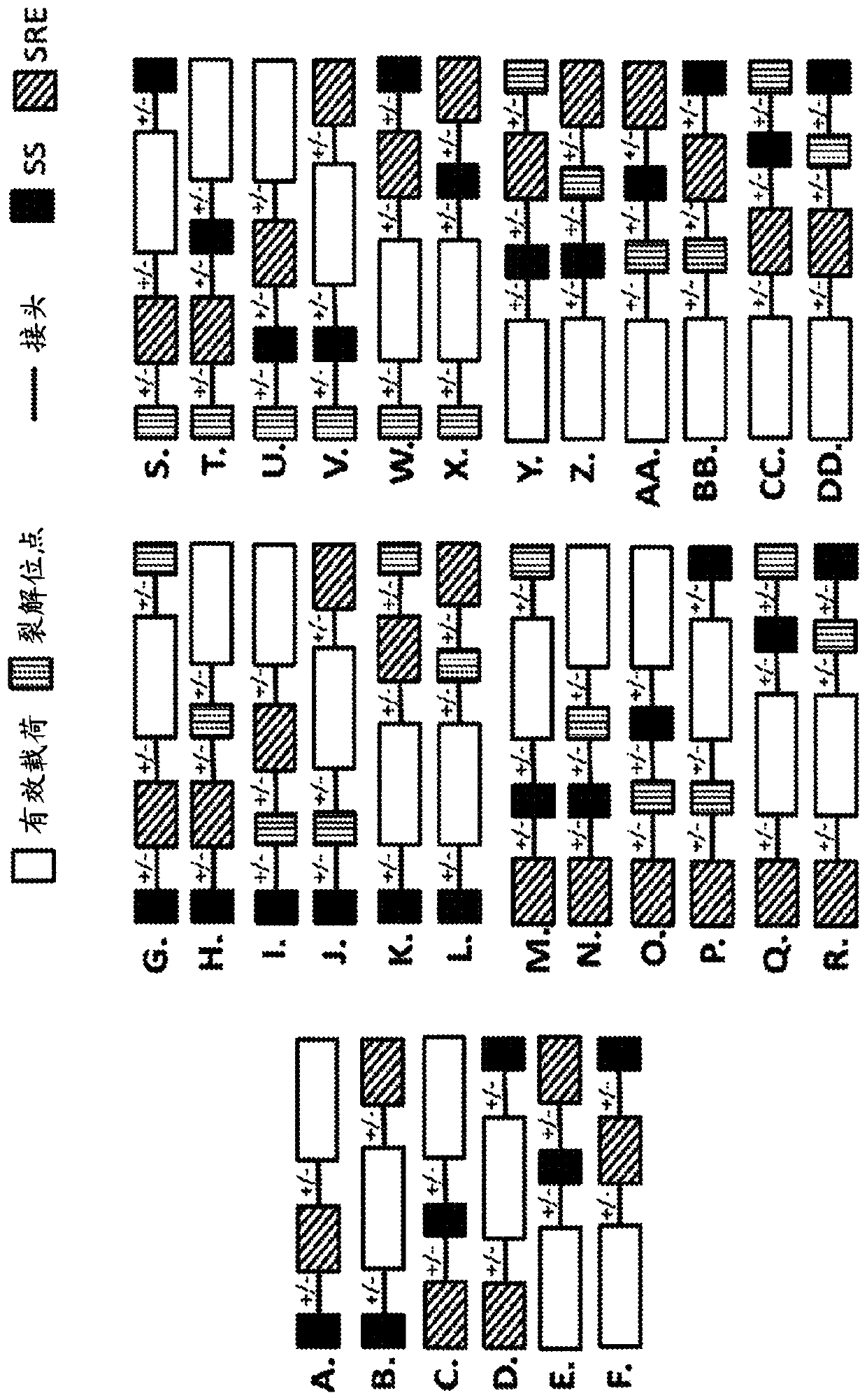
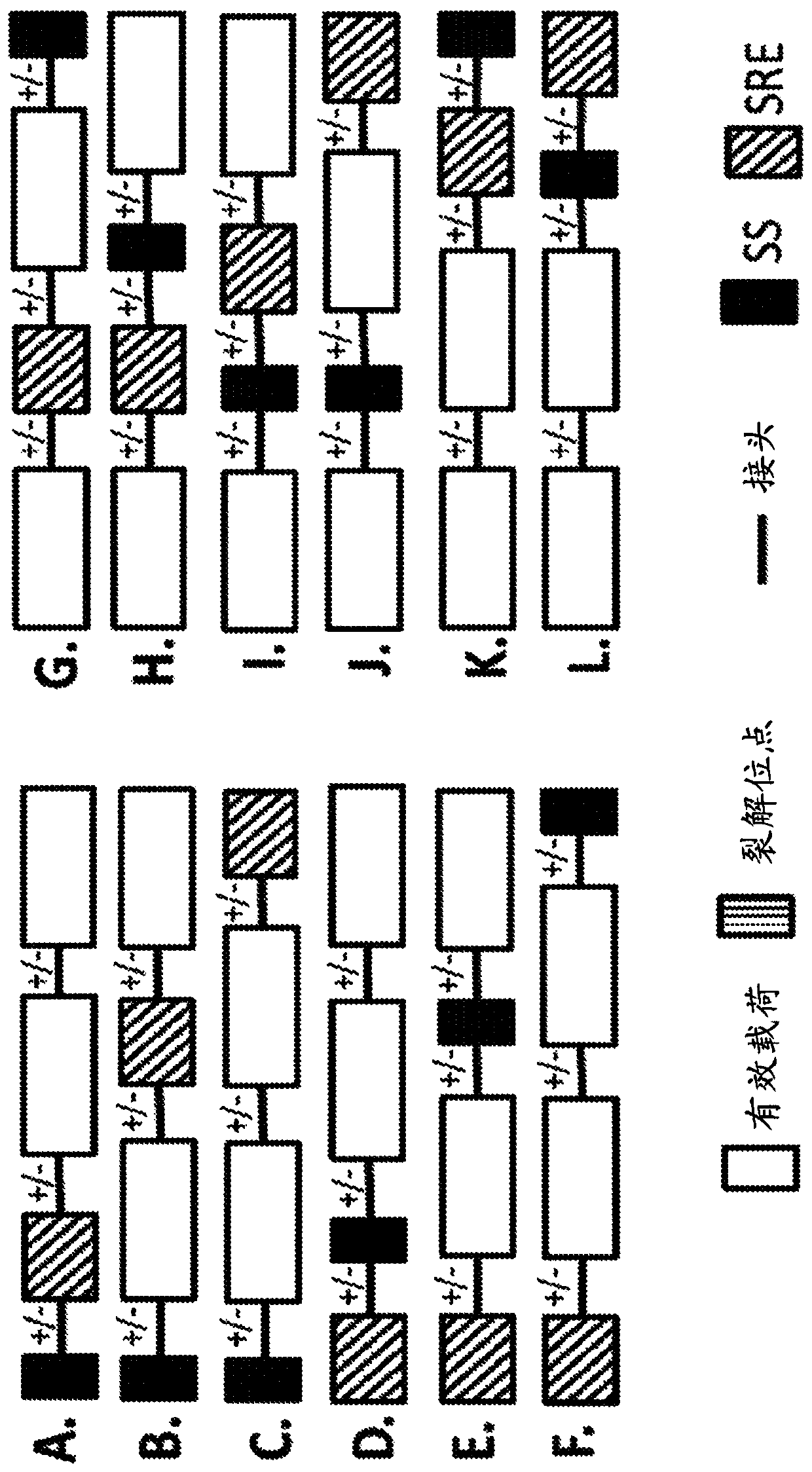
Pde5 compositions and methods for immunotherapy
A composition and immune response technology, applied in biochemical equipment and methods, chemical equipment and methods, for targeting specific cell fusion, etc., can solve the problem of lack of effective anti-tumor response and unsatisfactory therapeutic efficacy of immunotherapy schemes And other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0315] The preparation of antibodies, whether monoclonal or polyclonal, is known in the art. Techniques for producing antibodies are well known in the art and are described, for example, in Harlow and Lane "Antibodies, A Laboratory Manual", Cold Spring Harbor Laboratory Press, 1988; Harlow and Lane "Using Antibodies: A Laboratory Manual" Cold Spring Harbor Laboratory Press, 1999 and "Therapeutic Antibody Engineering: Current and Future Advances Driving the Strongest Growth Area in the Pharmaceutical Industry" Woodhead Publishing, 2012.
[0316] Antibodies and fragments and variants thereof as described herein can be produced using recombinant polynucleotides. In one embodiment, the polynucleotide has a modular design to encode at least one of an antibody, fragment or variant thereof. As a non-limiting example, a polynucleotide construct may encode any of the following designs: (1) heavy chain of antibody, (2) light chain of antibody, (3) heavy and light chain of antibody, (4)...
Embodiment 1
[1201] Example 1. Generation of novel ligand-responsive DDs by mutagenesis screening
[1202] Research design
[1203] To engineer constructs that exhibit ligand-dependent stability, candidate ligand-binding domains (LBDs) were selected, and a cell-based screen using yellow fluorescent protein (YFP) as a protein stability reporter was designed to identify the Mutants of candidate LBDs with the following desirable characteristics of the destabilizing domain: lower protein levels (i.e., low basal stability) in the absence of ligand for the LBD, large dynamic range, robust and predictable dose-response behavior , and fast degradation kinetics (Banaszynski et al., (2006) Cell; 126(5):995-1004). Candidate LBDs bind to desired ligands rather than endogenous signaling molecules.
[1204] A candidate LBD sequence (as a template) is first mutated using a combination of nucleotide analog mutagenesis and error-prone PCR to generate a library of mutants based on the template candidate...
Embodiment 2
[1212] Example 2. Novel DDs obtained from human PDE5 by site-directed mutagenesis
[1213] To identify novel destabilizing domain mutations, mutagenesis PCR was performed on the open reading frame of the human PDE5 catalytic domain (SEQ ID NO. 3) using unnatural nucleotides. The mutant library was ligated at the C-terminus in frame with the AcGFP reporter and cloned into the pLVX-IRES-Puro vector. The lentiviral library was then used to infect HEK 293T cells. Cells were selected with puromycin and the library was screened using the screening strategy described in Example 1. DNA was extracted from cell pools, cloned into vectors, and transformed into E. coli. Individual clones were sequenced and cloned in-frame at the C-terminus into pLVX.IRES puro with linker GGSGGGSGG (SEQ ID NO. 77) and AcGFP. The catalytic domain of wild-type hPDE5 was also cloned into pLVX.IRES.Puro and used as a control. HEK293 cells were transduced with individual clones and selected with puromycin...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com