Formulations comprising a nucleic acid in a high concentration
A nucleic acid and preparation technology, applied in the field of defibrillated glycosides, DNA and RNA vaccines, and can solve problems such as incompatibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
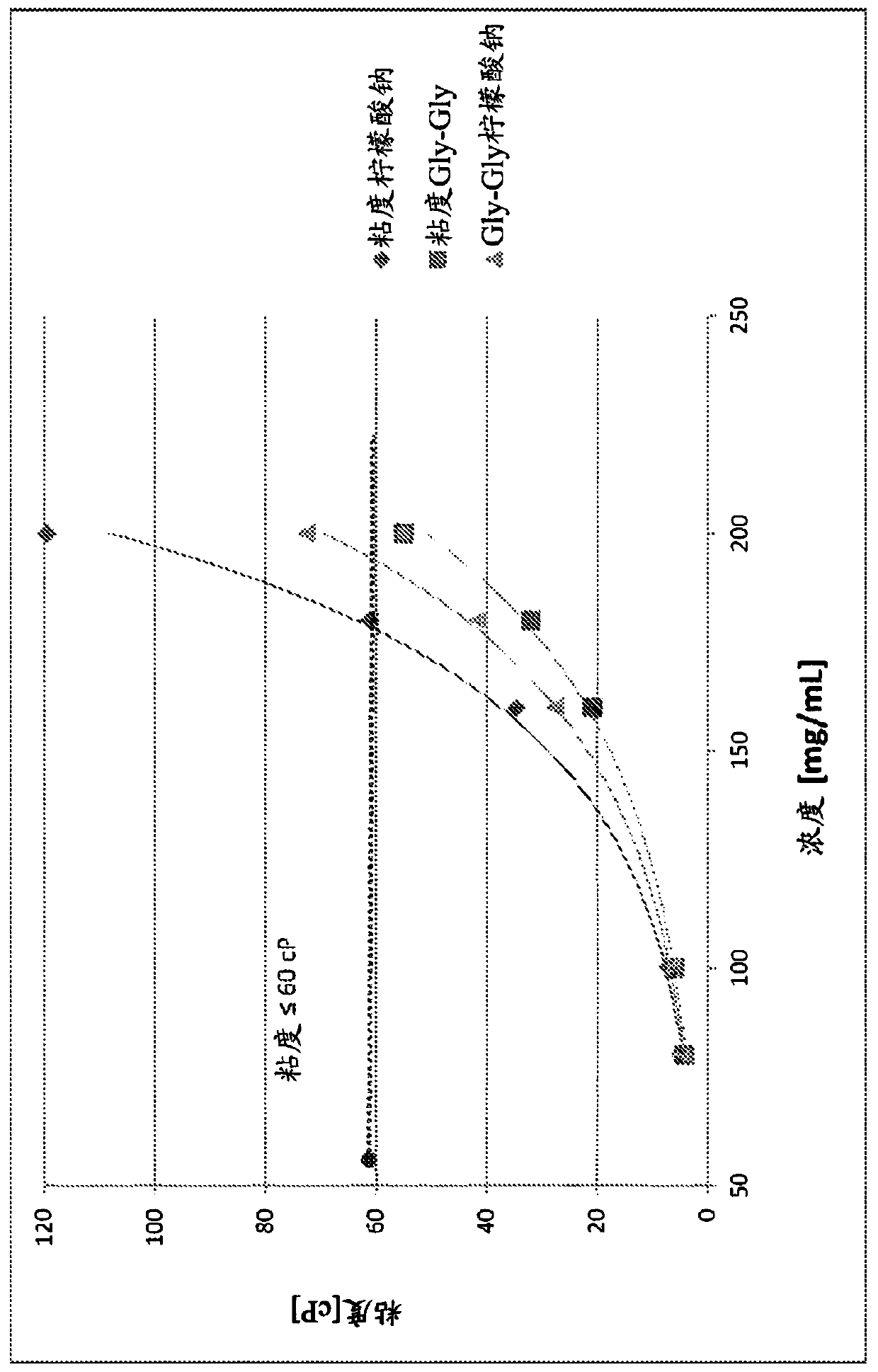
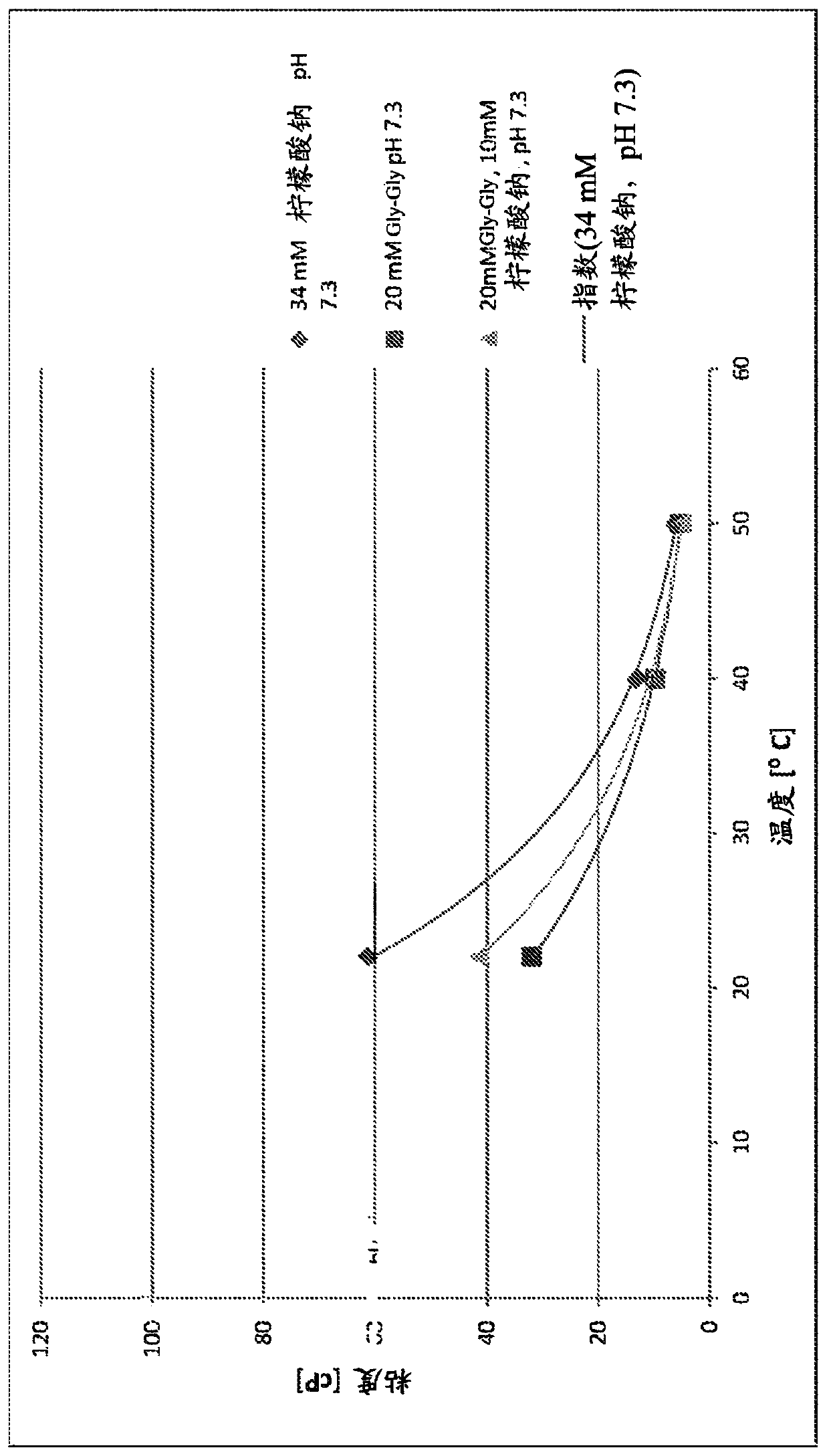
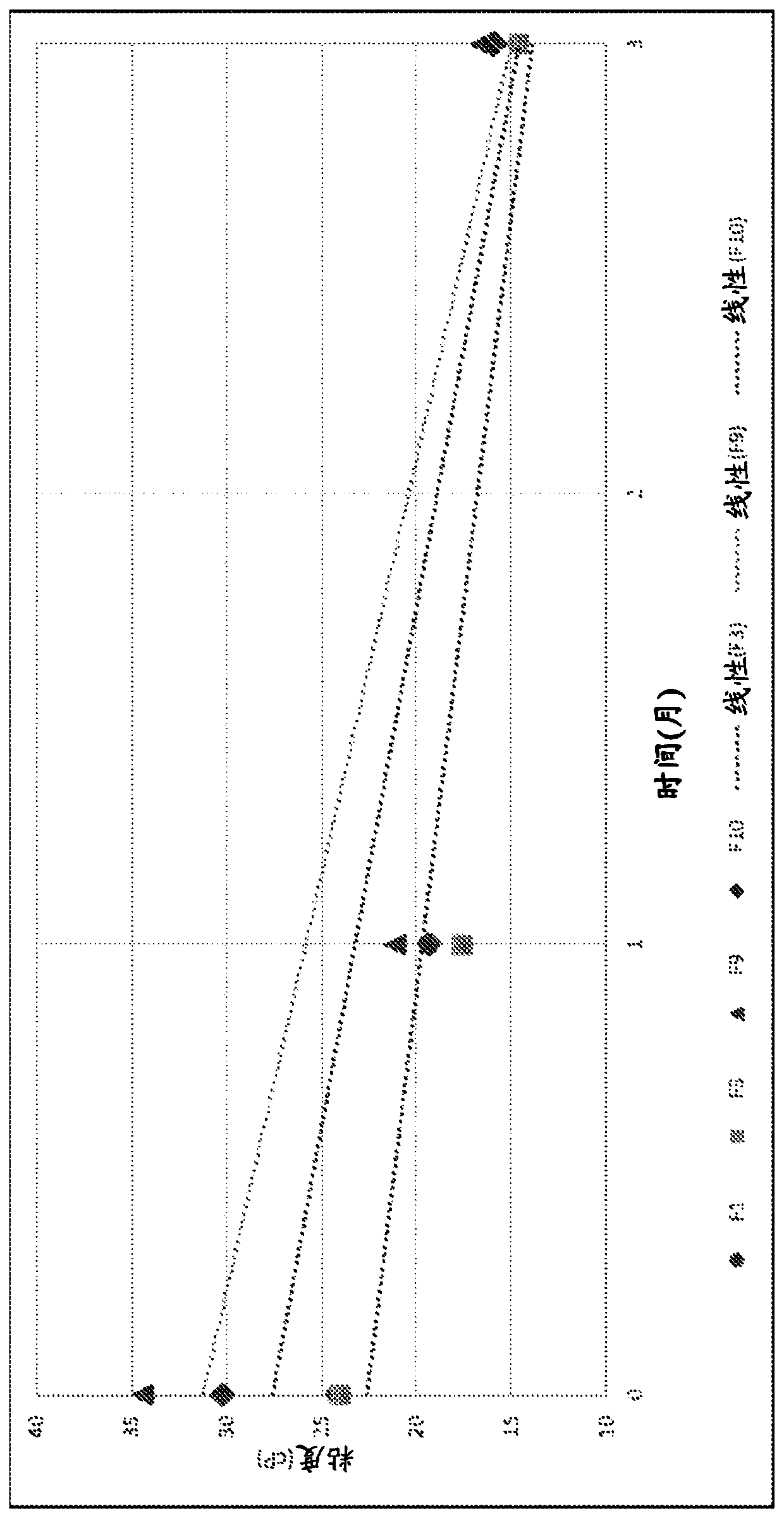
[0139] It is shown in Example 1 that increasing defibrotide concentration increases both viscosity and osmolarity. For pharmaceutical formulations for parenteral administration, low viscosity and / or isotonicity are important. To identify buffers or excipients that may reduce the viscosity and / or osmolarity of defibrotide formulations, a 200 mg / mL defibrotide formulation was performed on various buffers and excipients, including GRAS excipients. Wide plank screening.
[0140] As shown in Table 1 below, test formulations were prepared to target 200 mg / mL.
[0141] Table 1 : Defibrotide formulations using various buffers and excipients
[0142]
[0143]
[0144] Table 2: Solution Viscosity and Osmolality of Defibrotide in Gly-Gly-Containing Buffers as a Function of Product Concentration Compared to Sodium Citrate
[0145]
[0146]
[0147]
[0148]
[0149]
[0150]
[0151] as in Tables 1 and 2 and Figure 1B , 1C As shown in the figure in and 2A, ...
Embodiment 6
[0178] 6.6 Example 6 - Pharmacokinetics of Nucleic Acid Formulations Using Various Routes of Administration
[0179] 6.6.1 Example 6.1 - Intravenous (IV) infusion, IV bolus injection, subcutaneous (SC) injection and intramuscular injection of defibrotide (IM) injection
[0180] When administered to male Gottingen pigs at doses via a single 2-hour intravenous (IV) infusion, IV bolus injection, subcutaneous (SC) injection, intramuscular (IM) injection, or oral (PO) gavage The pharmacokinetics (PK) of various defibrotide preparations were compared. Additionally, the bioavailability of various extravascular routes of administration was determined relative to IV infusion.
[0181] The male Gottingen pig or minipig is the industry standard for exploring subcutaneous delivery and is an accepted model for studying subcutaneous formulations and delivery options of defibrotide. As listed in Table 5 for treatment groups, each animal received a single administration of defibrotide...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Viscosity | aaaaa | aaaaa |
| Viscosity | aaaaa | aaaaa |
| Viscosity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com