A signal peptide mutant for increasing the secretion of heterologous protein, its construction method and application
A technology of heterologous protein and signal peptide, applied in the field of genetic engineering, can solve the problem of difficulty in large-scale production of heterologous protein, and achieve the effects of significant secretion expression, promoting secretion expression, and increasing expression amount.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0067] Example 1: Preparation of signal peptide mutants and their induction of foreign protein expression
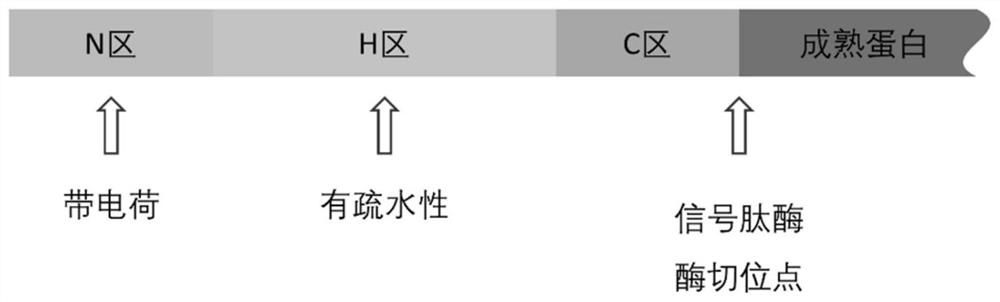
[0068] Using the alkaline protease derived from Bacillus clausii as the reporter gene, the signal peptide derived from Bacillus amyloliquefaciens was transformed to obtain a signal peptide mutant, which increased the secretion of alkaline protease in the host of Bacillus subtilis, in which the signal peptide Refer to the attached structural diagram figure 1 .
[0069] (1) Acquisition of the target gene
[0070] Target gene is alkaline protease gene aprE (alkaline protease amino acid sequence is shown in SEQ ID NO:1, and the nucleotide sequence of protease gene aprE is shown in SEQ ID NO:12, GenBank:FJ940727.1, the construction of target gene Reference patent literature: Invention patent with application number 201910332253.1 "Genetically engineered bacteria for efficient heterologous expression of alkaline protease and its construction method", in which the acquisition...
Embodiment 2
[0101] Example 2: Preparation of signal peptide mutants and their induction of foreign protein expression
[0102] Using the alkaline xylanase derived from Bacillus pumilus as the reporter gene, the signal peptide YwjE derived from Bacillus subtilis was transformed to obtain a signal peptide mutant, which increased the secretion of alkaline xylanase in the host of Bacillus subtilis, The schematic diagram of the signal peptide structure refers to the attached figure 1 .
[0103] (1) Acquisition of the target gene alkaline xylanase gene:
[0104] The target gene alkaline xylanase gene (the amino acid sequence of alkaline xylanase is shown in SEQ ID NO: 5, the nucleotide sequence of alkaline xylanase gene is shown in SEQ ID NO: 13, 687bp, GenBank: KU301789.1) was synthesized by Suzhou Jinweizhi Co., Ltd.
[0105] Construction of recombinant alkaline xylanase genetically engineered bacteria:
[0106] The plasmid containing the alkaline xylanase gene was double digested (BamHI-...
Embodiment 3
[0127] Using the cutinase derived from Fusarium solani as the reporter gene, the signal peptide YjiA derived from Bacillus subtilis was transformed to obtain a signal peptide mutant, which can increase the secretion of cutinase in the host of Bacillus subtilis, and the structure diagram of the signal peptide is referred to attached figure 1 .
[0128] (1) Obtaining the target gene:
[0129] The target gene cutinase gene (the amino acid sequence of cutinase is shown in SEQ ID NO: 8, the nucleotide sequence of cutinase gene is shown in SEQ ID NO: 14, GenBank: M29759.1) was synthesized by Suzhou Jinweizhi Co., Ltd.
[0130] Construction of recombinant cutinase genetically engineered bacteria:
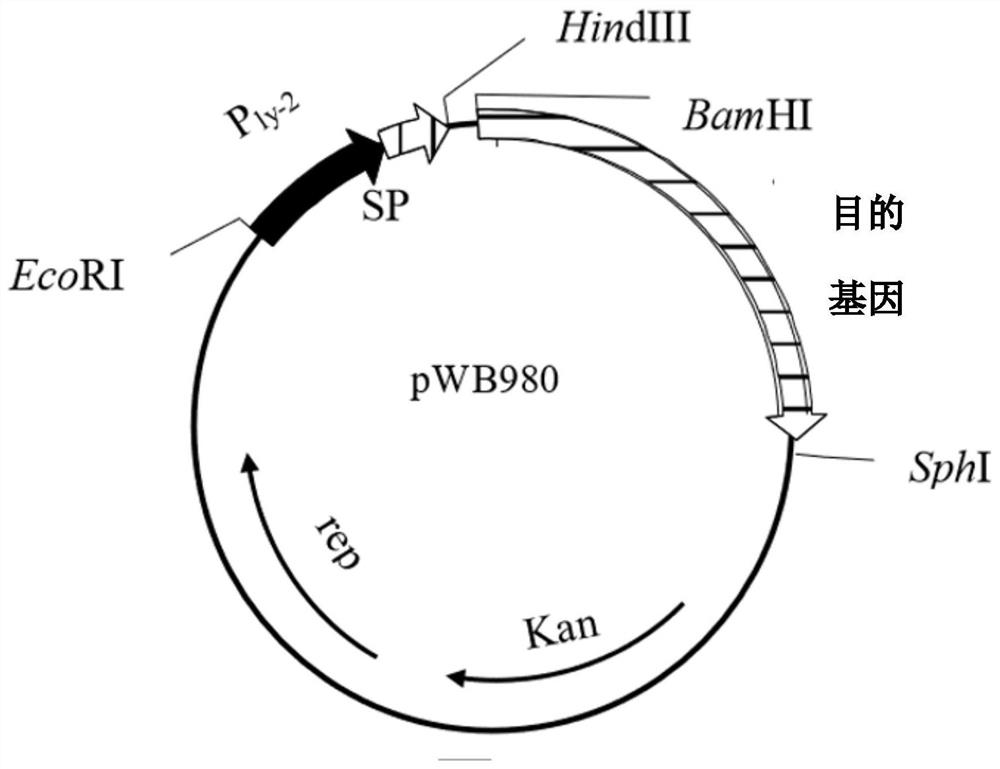
[0131] The plasmid containing the cutinase gene was double digested with (BamHI-SphI), and the 2770bp band was recovered by cutting the gel, and connected to the pWB980 expression vector (such as figure 2 shown), transformed into Bacillus subtilis WB600. Reference patent for the expre...
PUM
| Property | Measurement | Unit |
|---|---|---|
| hydrophobicity | aaaaa | aaaaa |
| hydrophobicity | aaaaa | aaaaa |
| hydrophobicity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com