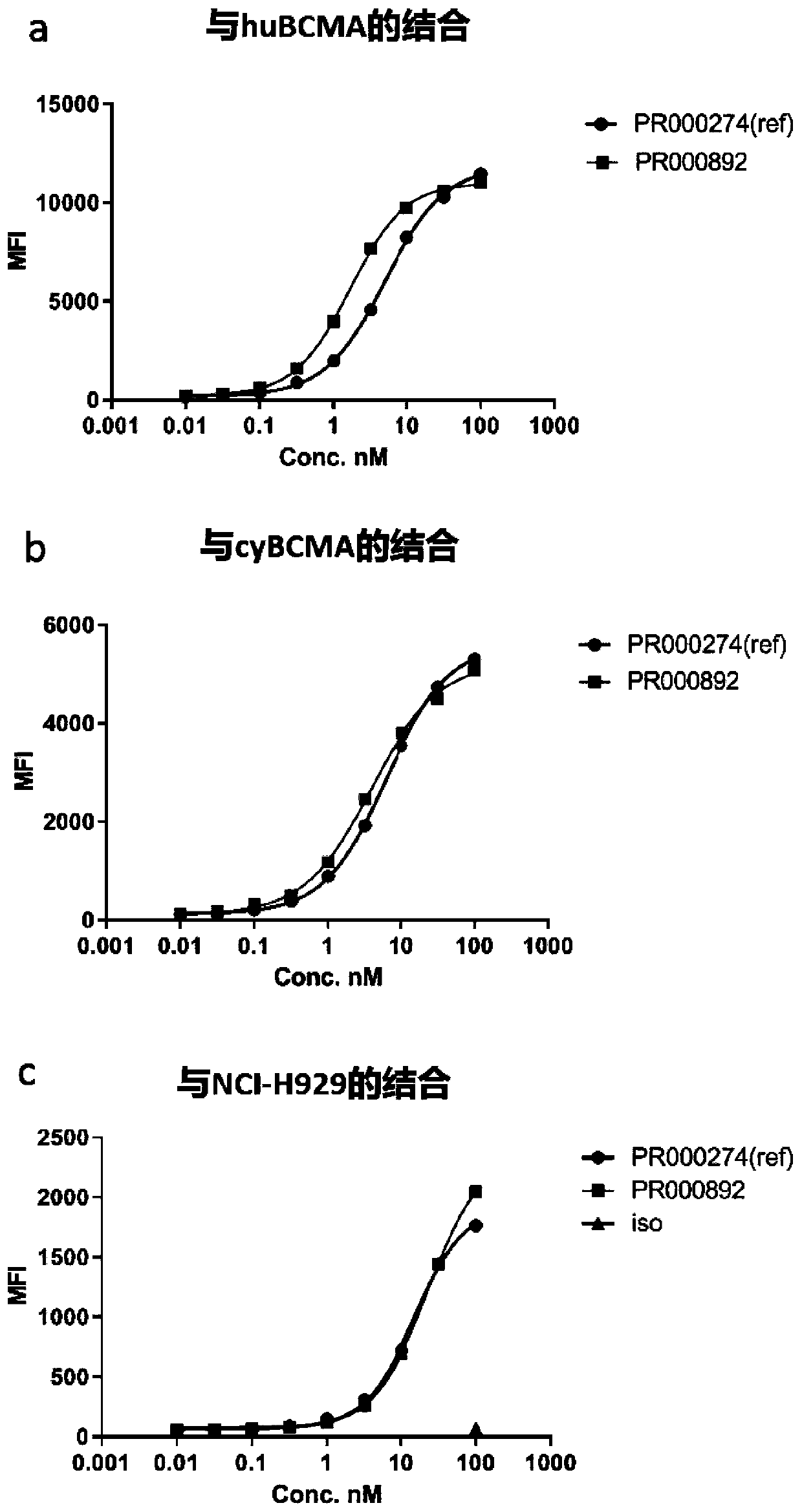
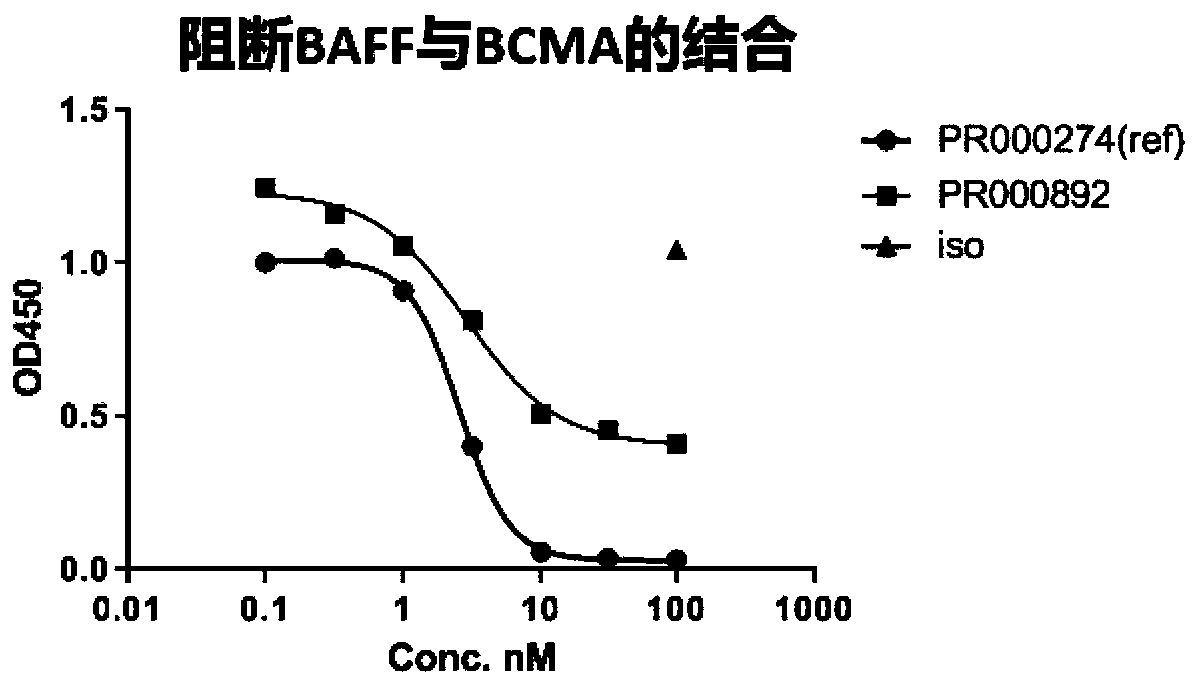
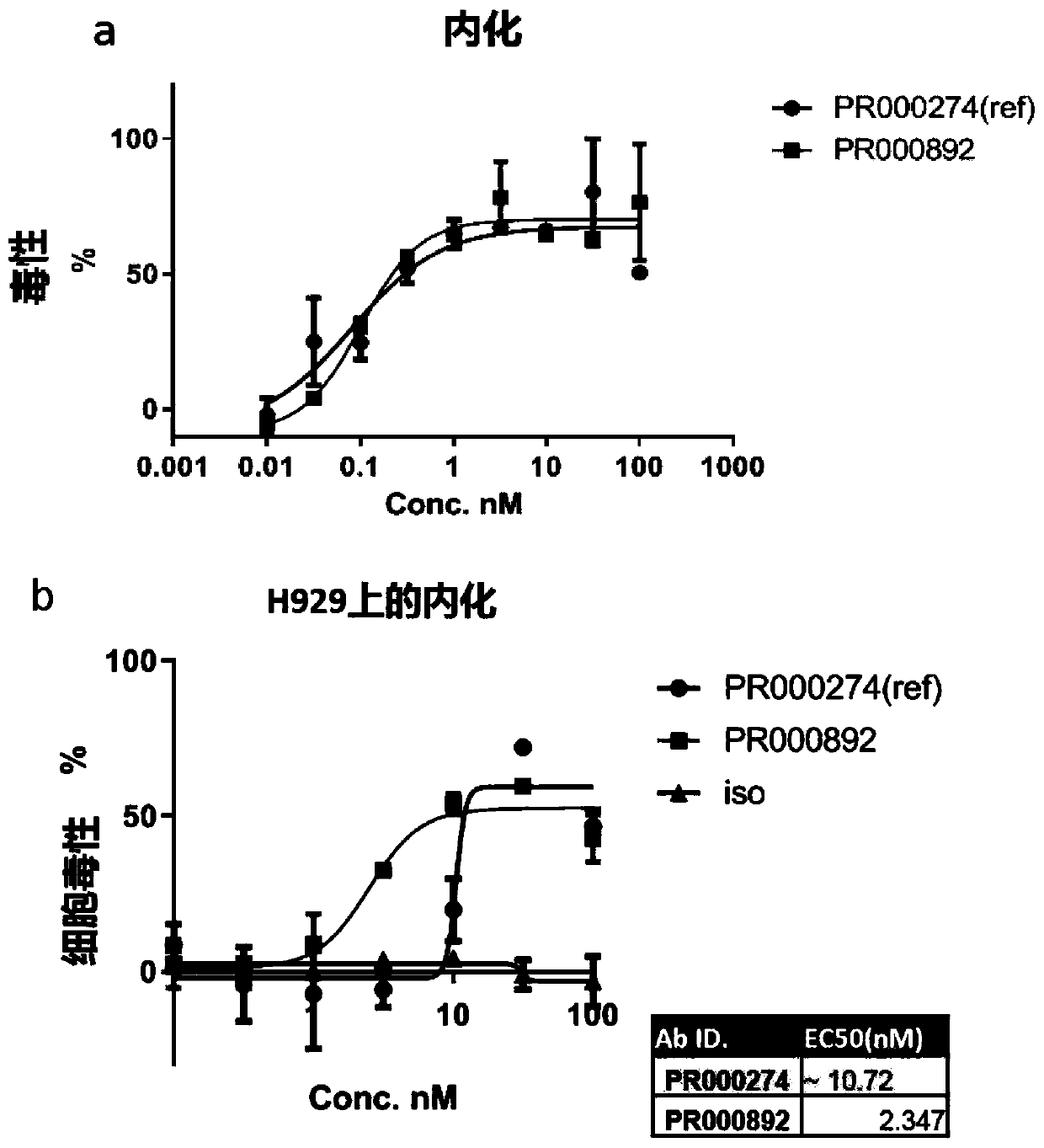
BCMA binding protein and preparation method and application thereof
A technology for binding proteins and amino acids, applied in the field of BCMA binding proteins and their preparation, which can solve the problems of weak internalization and weak binding
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053] Embodiment 1 Antigen preparation, mouse immunization and hybridoma preparation
[0054] 1. Antigen Preparation
[0055] (ACRO cat.no:BCA-C52H7)
[0056] supplier name NCBI ID Cat# Acrobio huBCMA-ECD-Fc Q02223 BCA-H522y Acrobio BCMA-His BCA-C52H7 Acrobio CynoBCMA-Fc G7NPN8 BCA-C5253 Acrobio CynoBCMA-His BCA-C5225 Acrobio BAFF-his-avitag Q9Y275 BAF-H82Q2
[0057] 2. Immunity
[0058] Fully human anti-BCMA antibodies were identified from hybridomas generated from H2L2 mice immunized with BCMA-ECD-Fc protein (Harbor Pharmaceuticals, EP2379727B1). For the first injection, 50 μg of the above fusion protein was immunized with CFA as an immune adjuvant, and then 25 μg protein and Ribi adjuvant (Sigma-Aldrich; Sigma AdjuvantSystem; Catalog Number S6322) and strengthened 7 times. On days 50, 78, and 107, blood was collected for testing, and the binding affinity of mouse serum was detected by FACS using ...
Embodiment 2
[0062] Example 2 Antibody Screening and Sequencing
[0063] 1. ELISA screening
[0064] Freshly prepared hBCMA ECD-Fc protein or hFc at 1 μg / ml in PBS was added to 96-well plates (Corning 9018), coated overnight at 4°C, then discarded and washed 3 times with PBST. Plates were blocked with 5% milk for 2 hours at room temperature and washed 3 times with PBST. 100 μl / well of hybridoma supernatant was added and incubated at room temperature for 1 hour, then washed 3 times with PBST. 100 μl / well of secondary antibody was added and incubated for 1 hour at room temperature, followed by washing. Add 100 μl / well TMB to the plate and incubate at room temperature for 15 min, then stop and read.
[0065] 2. FACS screening
[0066] For flow cytometry screening, adherent cells were digested with TypLE (CAT#12605010, Gibco) for 3 minutes at 37°C and the digestion was terminated with complete medium containing 10% FBS. The cells were washed and counted with FACS buffer (CAT#14190250, Gib...
Embodiment 3
[0077] Example 3. Antibody Production, Purification and Validation
[0078] 1. Recombinant Antibody Production and Purification
[0079] After obtaining the light and heavy chain variable domain sequences encoding antibody molecules, conventional recombinant DNA technology can be used to combine the light and heavy chain variable domain sequences with the corresponding human antibody light and heavy chain constant domain sequences. Fusion expression to obtain recombinant antibody molecules. In this example, the antibody heavy chain variable domain sequence (VH) was genetically synthesized and cloned into a mammalian cell expression plasmid vector encoding the human IgG1 antibody heavy chain constant domain sequence to encode the full-length IgG1 antibody. heavy chain. The antibody light chain variable domain sequence (VL) was genetically synthesized and cloned into a mammalian cell expression plasmid vector encoding the human antibody Ig kappa light chain constant domain seq...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com