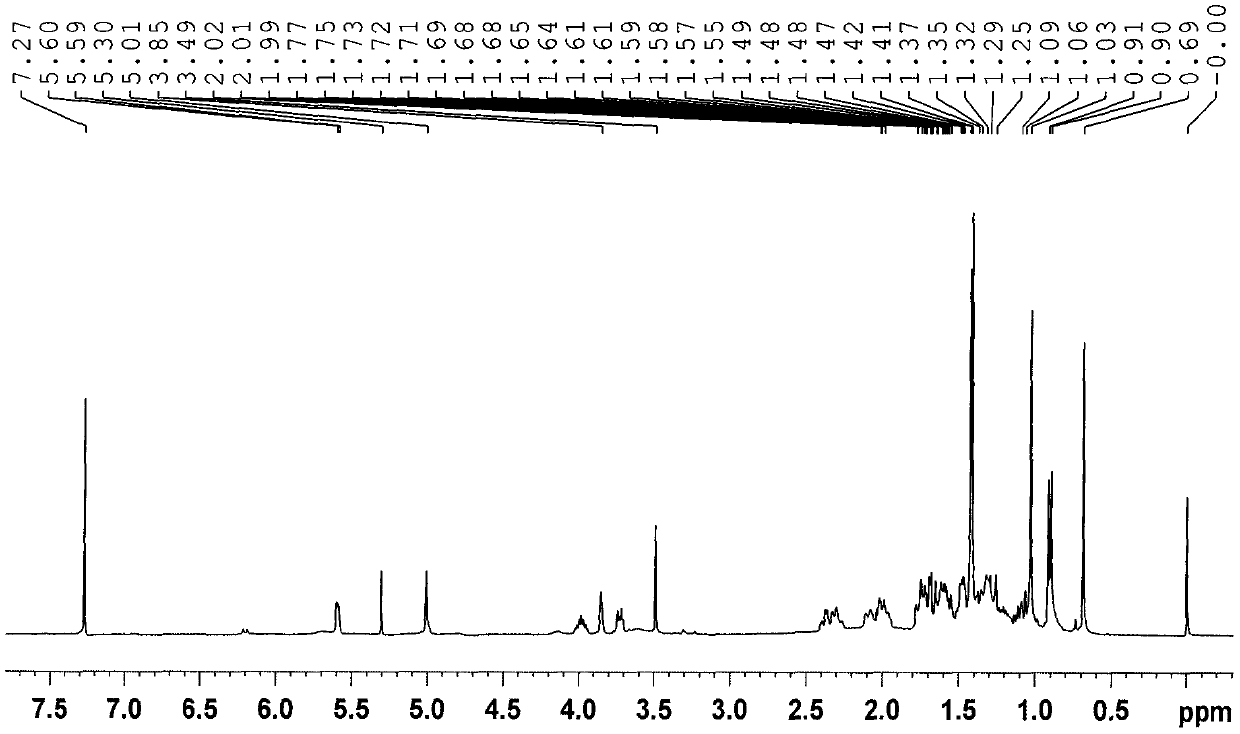
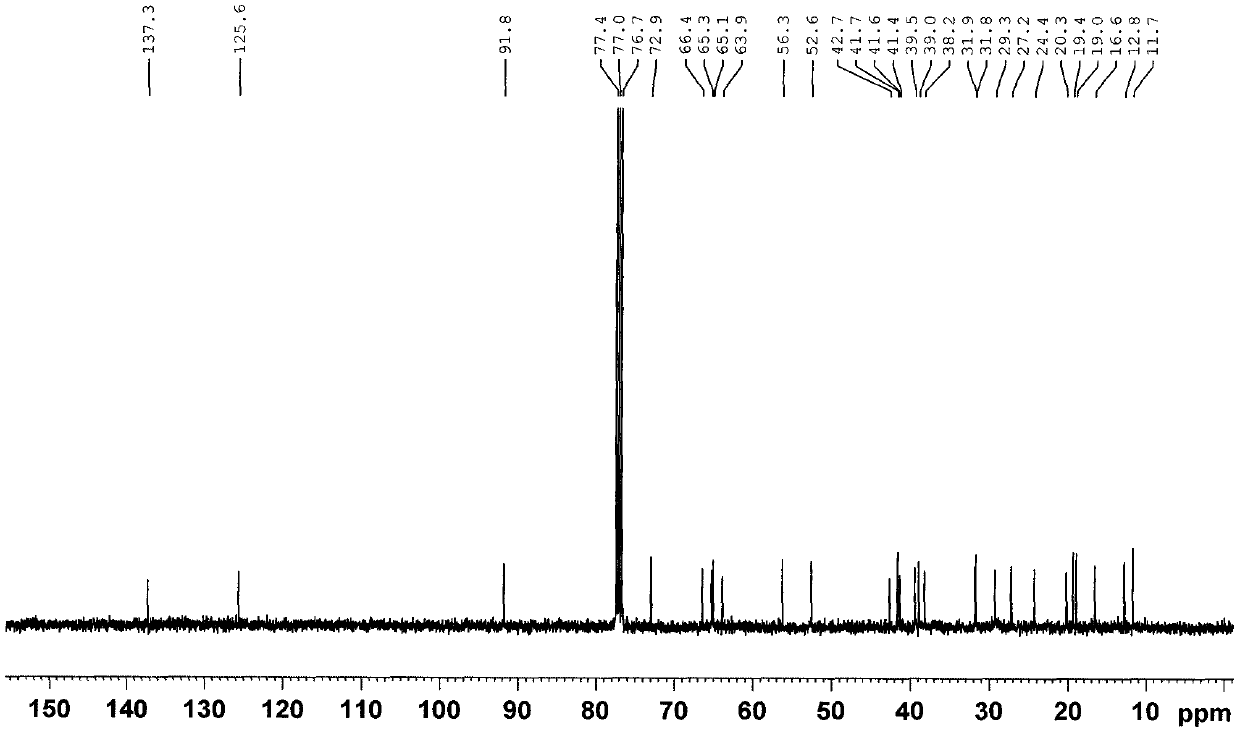
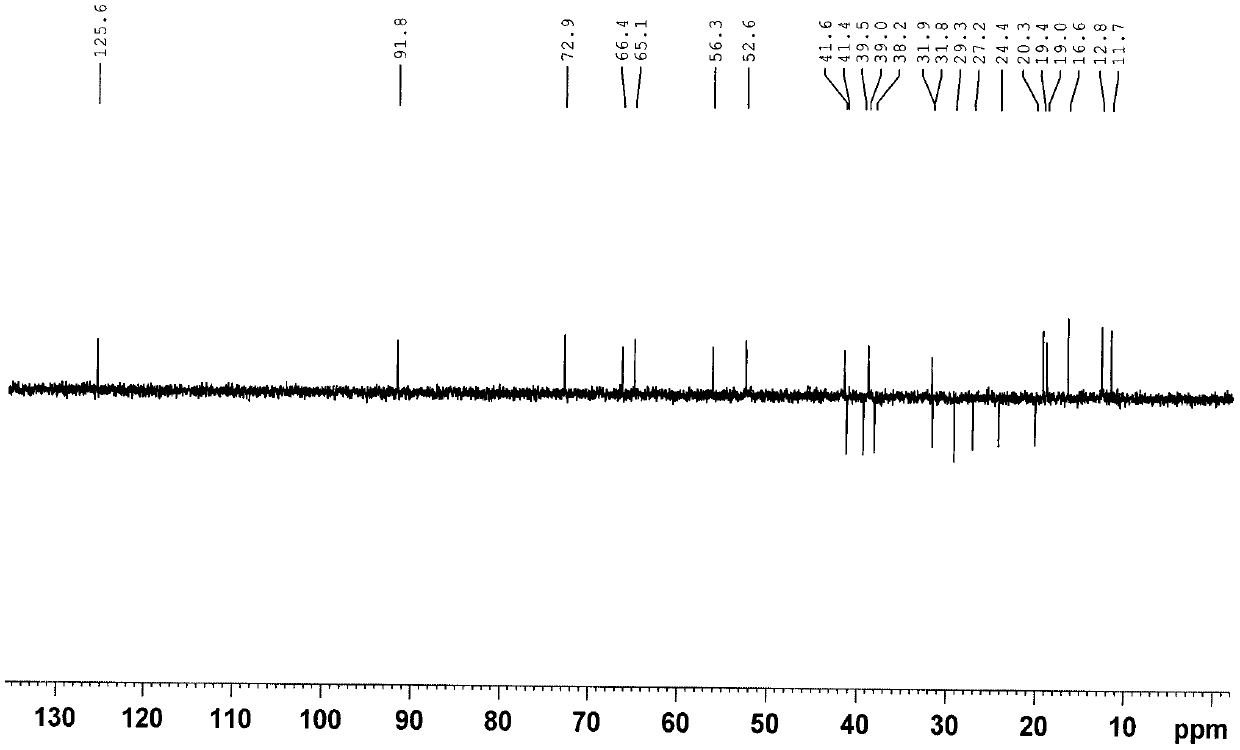
Seven withanolides compounds derived from cape gooseberries as well as preparation method and application of the withanolides compounds
A technology of ester compounds and compounds, applied in the field of medicine, can solve problems such as tissue surrounding cells and cell damage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] (1) 10 kg of the whole plant of Lanternia is extracted with methanol for 3 times (amount of 3 × 60 L), and the extract is recovered under reduced pressure to obtain a crude extract;
[0050] (2) The methanol extract obtained in step (1) is added with water to make a suspension, and extracted with ethyl acetate to obtain the ethyl acetate extract;
[0051] (3) Step (2) is separated by silica gel column chromatography, followed by petroleum ether: acetone 100:0, 100:2, 100:4, 100:7, 100:10, 100:14, 100:19, 100: 25, eluted at 100:33;
[0052] (3) Petroleum ether obtained in the above step (2): acetone 100: 2~100: 25 fractions are separated by medium pressure liquid chromatography (MPLC), using methanol / water 6: 4~9: 1 as mobile phase gradient Elution;
[0053] (4) The methanol / water (7:3) fraction obtained in the above step (3) was separated by HPLC-RI, and was eluted with methanol / water 60:40~90:10 as mobile phase to obtain compound 1 (yield 0.001%) ), 2 (0.001% yield)...
Embodiment 2
[0070] (1) 10.0 kg of the whole plant of Lanternia japonica was heated and extracted with ethanol for 3 times (amount of 3 × 48 L), and the extract was recovered under reduced pressure to obtain a crude extract;
[0071] (2) The ethanol extract obtained in step (1) is added with water to make a suspension, and extracted with ethyl acetate to obtain the ethyl acetate extract;
[0072] (3) Step (2) is separated by silica gel column chromatography, followed by petroleum ether: ethyl acetate 100:0, 100:2, 100:4, 100:7, 100:10, 100:15, 100:22, 100:30 elution;
[0073] (3) Petroleum ether obtained in the above step (2): ethyl acetate 100:2~100:22 fractions are separated by medium pressure liquid chromatography (MPLC), using methanol / water 6:4~9:1 as the mobile Phase gradient elution;
[0074] (4) The methanol / water (7:3) fraction obtained in the above step (3) was separated by HPLC-RI, and eluted with methanol / water 60:40~90:10 as the mobile phase to obtain 1 (yield 0.002%) , 2 (...
Embodiment 3
[0077] (1) 10.0 kg of the whole plant of Lanternia is extracted 3 times with acetone (amount of 3 × 60 L), and the extract is recovered under reduced pressure to obtain a crude extract;
[0078] (2) The acetone extract obtained in step (1) is added with water to make a suspension, and extracted with dichloromethane to obtain the dichloromethane extract;
[0079] (3) Step (2) is separated by silica gel column chromatography, followed by petroleum ether: acetone 100:0, 100:2, 100:3, 100:5, 100:7, 100:10, 100:15, 100: 25 elution;
[0080] (3) The petroleum ether obtained in the above step (2): 100:2~100:25 fractions of acetone are separated by medium pressure liquid chromatography (MPLC), using methanol / water 7:3~9:1 as the mobile phase gradient Elution;
[0081] (4) The methanol / water (8:2) fraction obtained in the above step (3) is separated by HPLC-RI, and the new compound 1 (yield 0.001 %), 2 (0.001% yield), 3 (0.001% yield), 4 (0.002% yield), 5 (0.002% yield), 6 (0.002% y...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com