Acetaminophen-pregabalin combinations and methods of treating pain
A technology of acetaminophen and pregabalin, applied in the field of manufacturing pharmaceutical preparations, pain in subjects, and pharmaceutical compositions for pain treatment, which can solve problems such as poor respiratory function
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0111] Example 1: Effect of pH on the solubility of pregabalin and acetaminophen.
[0112] The solubility of pregabalin (PGB) and acetaminophen (APAP) in water was determined at different pH values at room temperature. Samples were prepared by adding acetaminophen at a concentration of 50 mg / mL and pregabalin at a concentration of 100 mg / mL in water. The pH is then adjusted to the desired range. The concentration of the active ingredient was determined by HPLC-UV.
[0113] As can be seen from Table 1, no significant difference in the solubility of acetaminophen was observed within the pH range of 4-7.
[0114] As shown in Table 2, the solubility of pregabalin is higher at pH 4, being about 31 mg / mL in the pH range of 5-7.
[0115]
[0116]
[0117]
[0118] When pregabalin and acetaminophen are co-dissolved in the aqueous vehicle, the concentration of pregabalin and acetaminophen in the aqueous vehicle can be increased. Solubility samples were prepared by adding...
Embodiment 2
[0121] Example 2: Stability of acetaminophen and pregabalin formulations.
[0122] To determine the stability of acetaminophen and pregabalin co-formulations, citrate buffer (10 mM), acetate buffer (10 mM) and phosphate buffer (10 mM) were housed in four separate manufacturing tanks , as shown in Table 4. Pregabalin, acetaminophen, and other excipients were added to each tank and filled with the required amount of water (water for injection) to obtain concentrations of 4.5 mg / mL and 10 mg / mL, respectively.
[0123] Then, readjust the pH of each tank using HCl or NaOH. The volume of the tank can be topped up with deoxygenated water.
[0124] The bulk solution was filled into 100 mL polypropylene bags with a target fill volume of 100 mL, then stoppered and sealed. The bag is then further packed in an aluminum bag together with an oxygen scavenger and sealed.
[0125] Samples were assayed and tested for impurities. Table 4 shows four different formulations of pregabalin and ...
Embodiment 3
[0145] Example 3: Pharmacokinetic parameters of acetaminophen-pregabalin combination formulations.
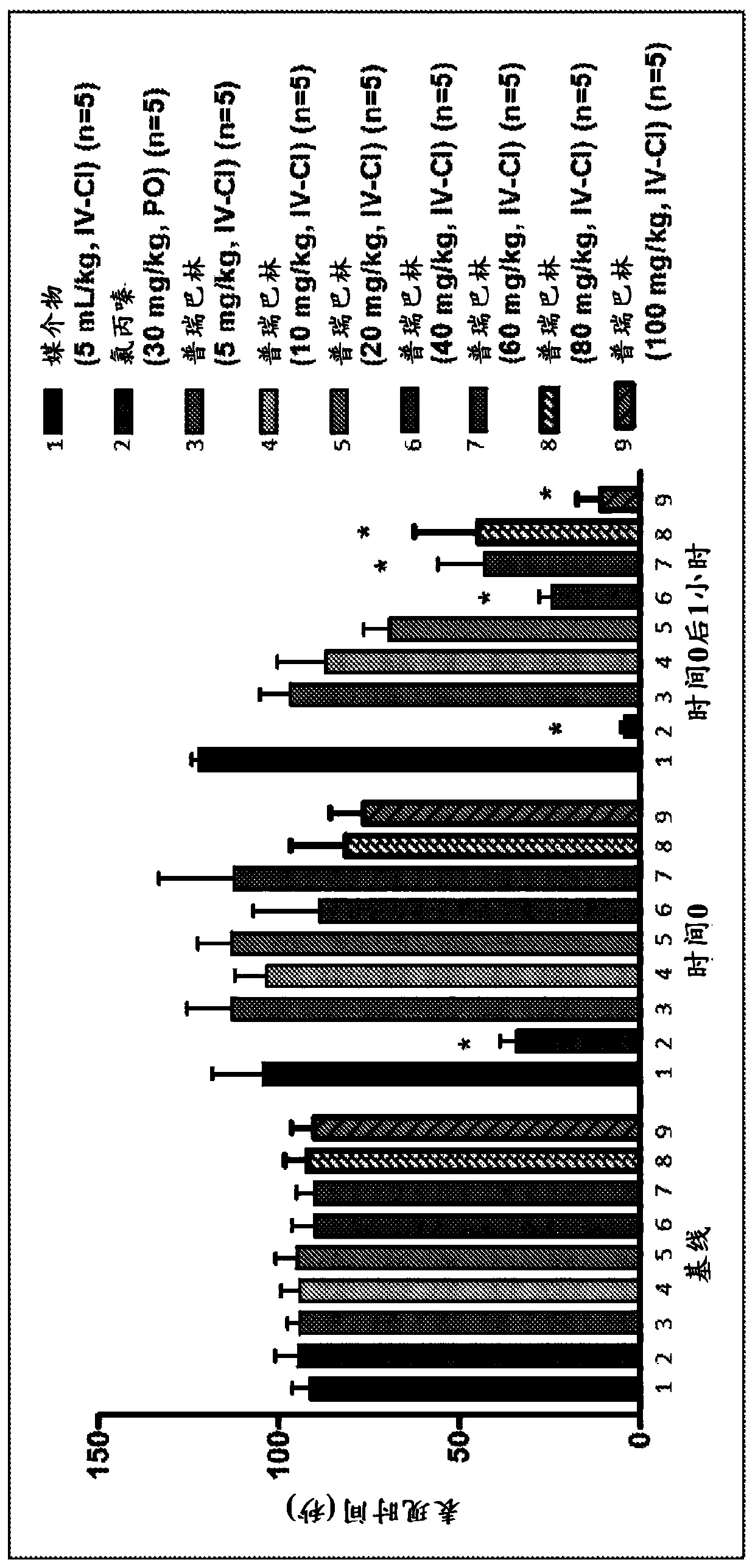
[0146] Formulations for testing were prepared by weighing pregabalin and / or acetaminophen as indicated in Table 10 in glass vials. The active ingredient was dissolved in 0.9% saline (20 mL) to the desired concentration. Mix the brine and vial ingredients with gentle agitation until a clear solution is obtained. The obtained clear solution was filtered through a sterile 0.45 μm PVDF membrane syringe filter before being used for further studies.
[0147]
[0148]
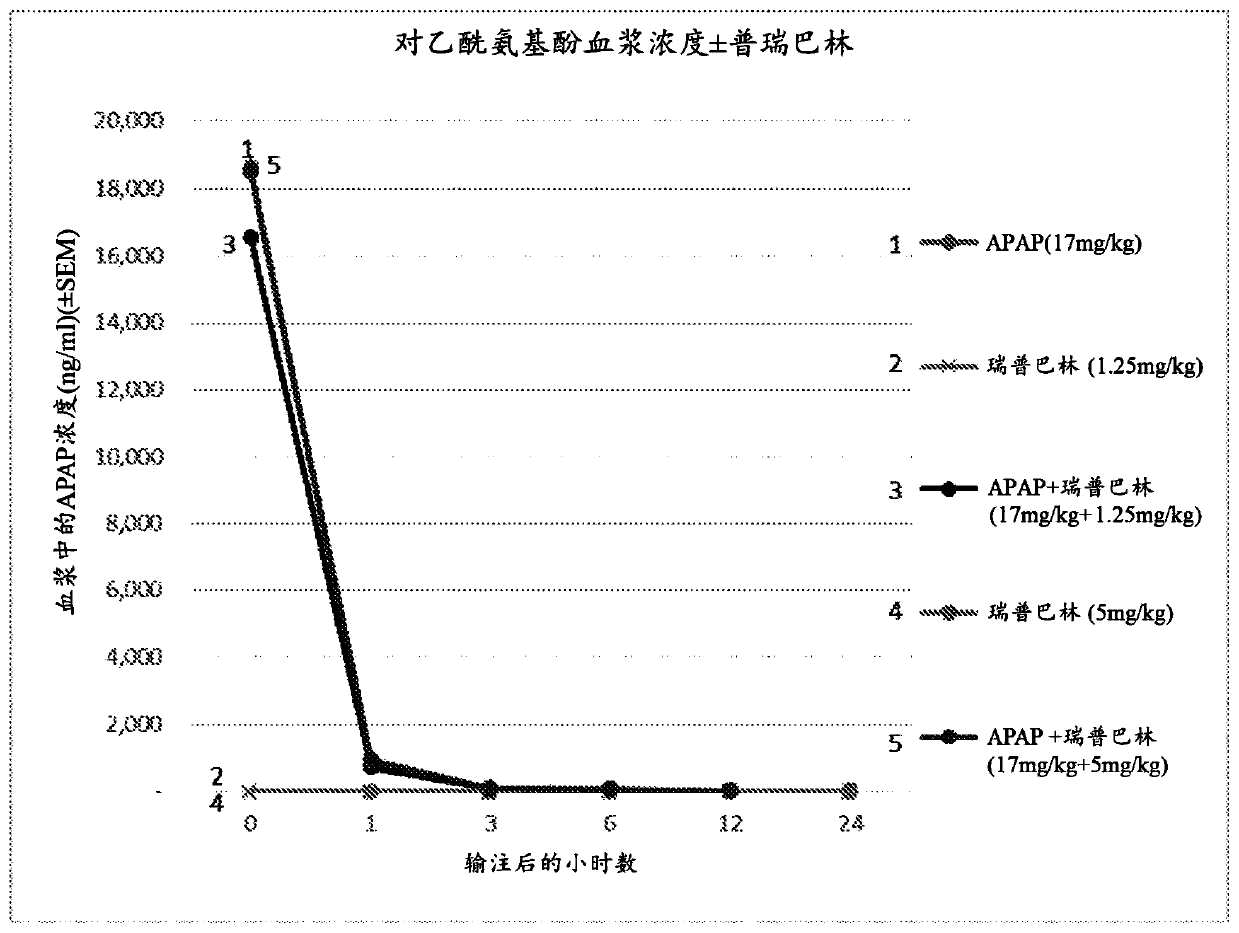
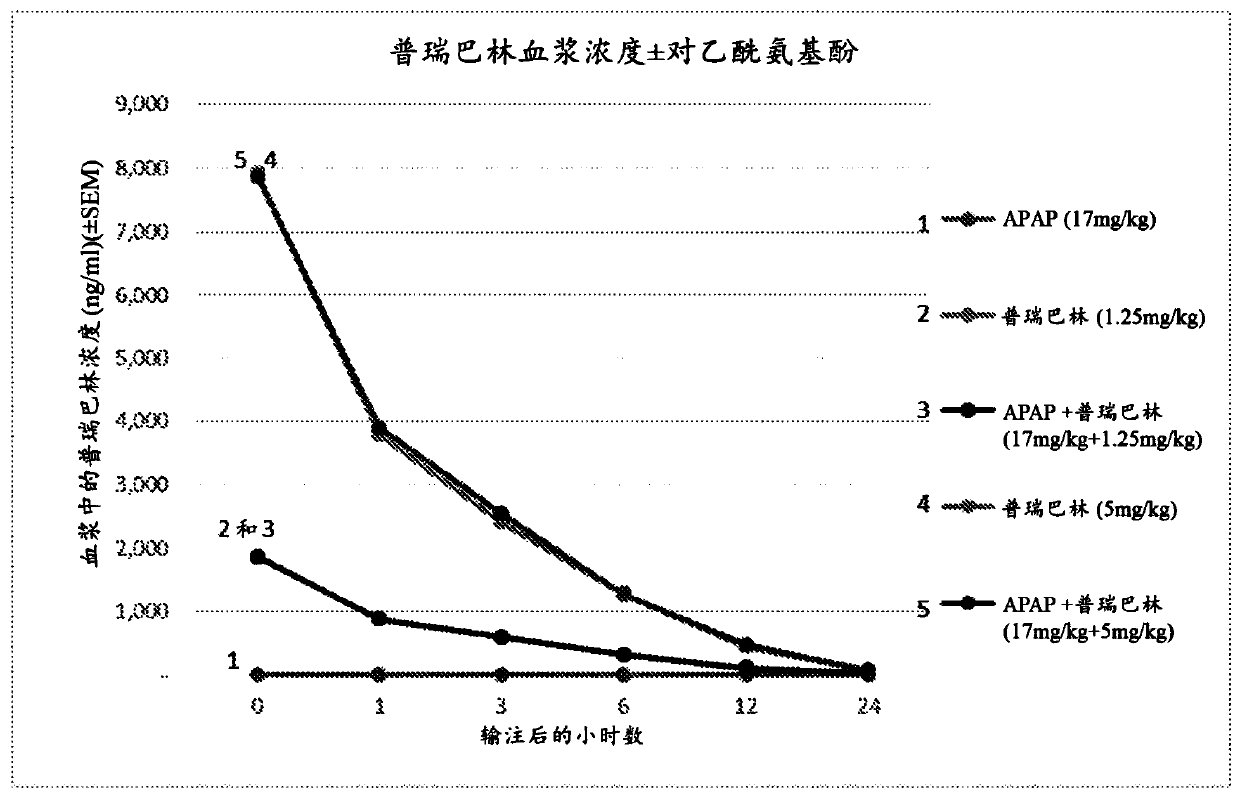
[0149] Carry out paracetamol, pregabalin and paracetamol and pregabalin combination preparation after 15min infusion in rat body, analyze pharmacokinetic parameter in the period of time of 24h (table 11, figure 1 - Acetaminophen; Table 12, figure 2 - pregabalin).
[0150] The presence or absence of pregabalin did not affect the pharmacokinetics of acetaminophen in rats (Table 11; figure 1 ). No changes i...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com