Sustained-release preparation for postoperative analgesia and preparation method thereof
A technology of sustained-release preparations and cosolvents, which can be used in non-central analgesics, medical preparations without active ingredients, and medical preparations containing active ingredients, etc., and can solve problems such as inconvenient management, difficult control, and harmful side reactions. , to achieve the effect of easy injection, reduce the frequency of medication, and avoid toxic and side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
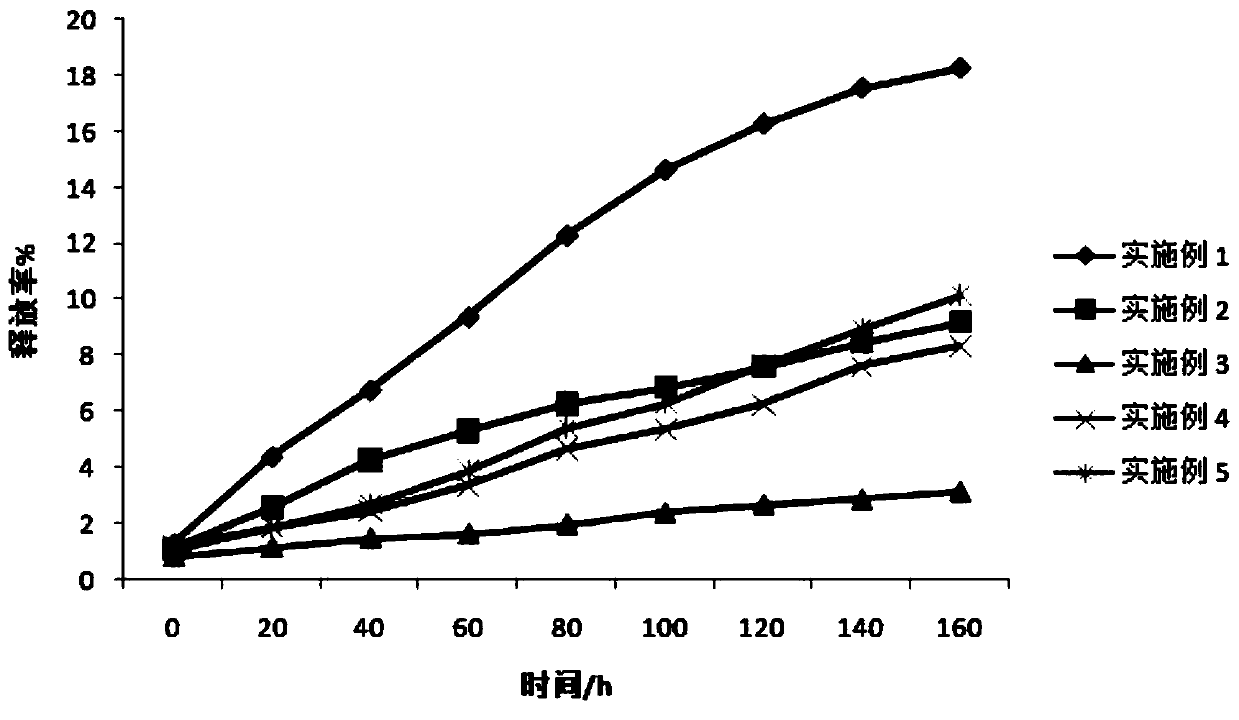
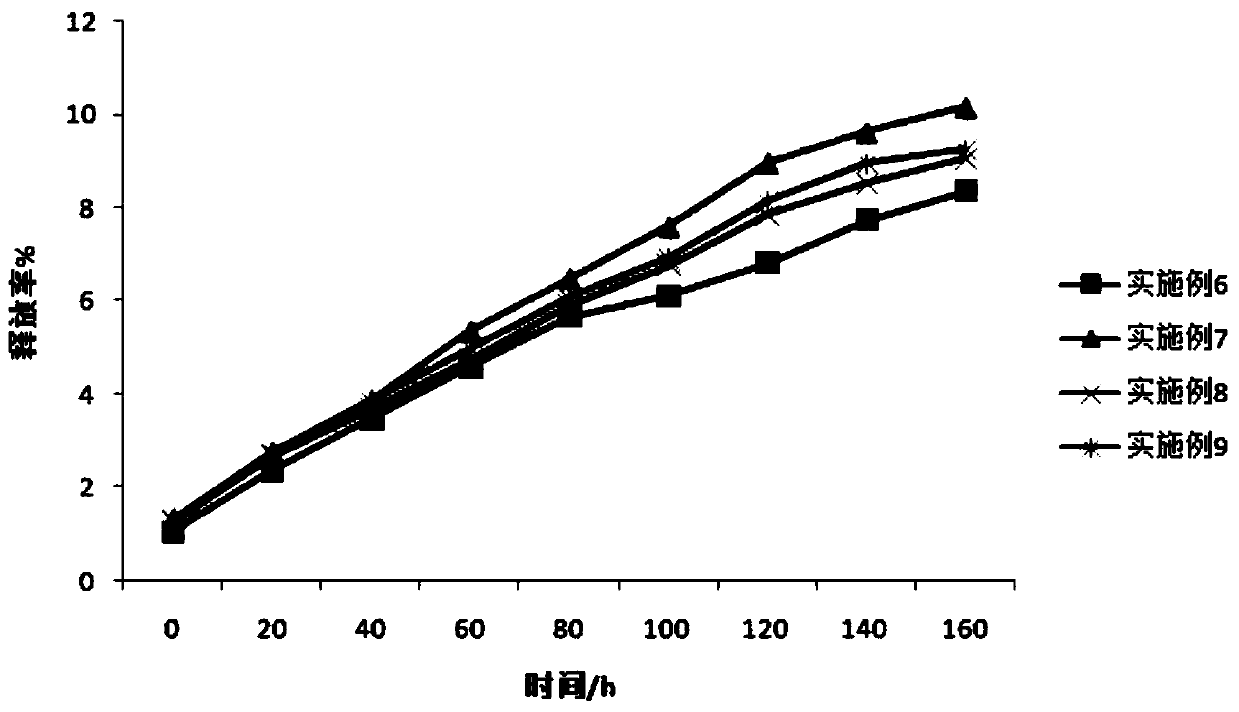
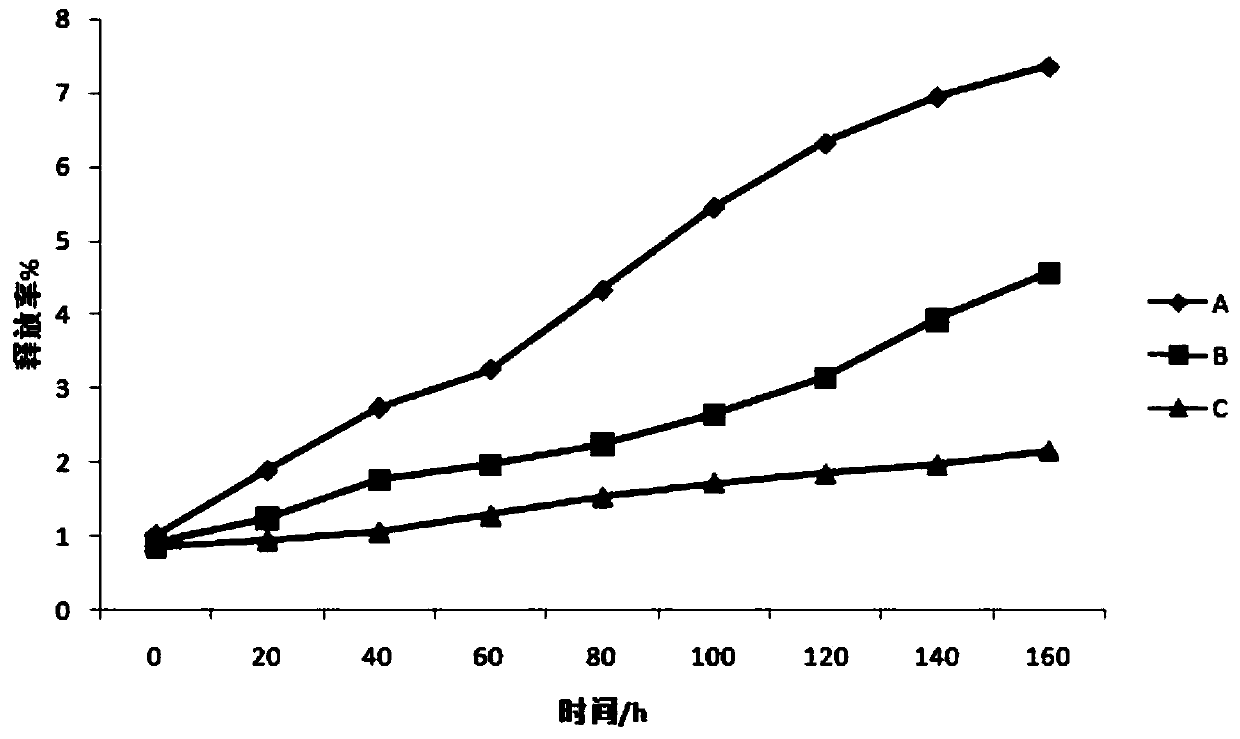
Image
Examples
Embodiment 1
[0029] A sustained-release preparation for postoperative analgesia, mainly made of the following raw materials:
[0030] A mixture of 120mg soybean phosphatidylcholine, 50mg diolein, 20mg poloxamer, 30mg absolute ethanol, 12mg cocaine, procaine and lidocaine local anesthetics.
[0031] The preparation method of the sustained-release preparation for postoperative analgesia is as follows:
[0032] Dissolve cocaine, procaine, and lidocaine local anesthetics in absolute ethanol at a ratio of 1:1:4, and mix well to obtain a drug solution; soybean phosphatidylcholine, glyceryl dioleate, porol Sham is heated and melted at 70°C to obtain a molten mixed solution; slowly add the molten mixed solution to the drug solution, vortex and mix, and place it at room temperature to obtain the liquid crystal for postoperative analgesia Gel sustained release formulation precursor formulation. Protect from light, seal, and store in a refrigerator at 4°C.
Embodiment 2
[0034] A sustained-release preparation for postoperative analgesia of this embodiment is mainly made of the following raw materials: 120mg soybean phosphatidylcholine, 50mg diolein, 20mg poloxamer 407, 30mg ethanol, 12mg cocaine, A mixture of lucaine and lidocaine local anesthetics.
[0035] The preparation method of the sustained-release preparation for postoperative analgesia is as follows:
[0036] Dissolve the local anesthetic mixture of cocaine, procaine and lidocaine in absolute ethanol at a ratio of 1:1:5.5, and mix well to obtain a drug solution; soybean phosphatidylcholine, glyceryl dioleate, Loxamer is heated and melted at 70°C to obtain a molten mixed solution; the molten mixed solution is slowly added to the drug solution, vortexed, and placed at room temperature to obtain the above-mentioned drug for postoperative analgesia. Liquid crystal gel sustained release formulation precursor formulation. Protect from light, seal, and store in a refrigerator at 4°C.
Embodiment 3
[0038] A sustained-release preparation for postoperative analgesia provided in this example is mainly made of the following raw materials: 120mg soybean phosphatidylcholine, 50mg diolein, 20mg poloxamer, 30mg ethanol, 12mg cocaine, A mixture of procaine and lidocaine local anesthetics.
[0039] The preparation method of the sustained-release preparation for postoperative analgesia is as follows:
[0040] Dissolve the local anesthetic mixture of cocaine, procaine and lidocaine in absolute ethanol at a ratio of 1:1:6, and mix well to obtain a drug solution; soybean phosphatidylcholine, glyceryl dioleate, Loxamer is heated and melted at 70°C to obtain a molten mixed solution; the molten mixed solution is slowly added to the drug solution, vortexed, and placed at room temperature to obtain the above-mentioned drug for postoperative analgesia. Liquid crystal gel sustained release formulation precursor formulation. Protect from light, seal, and store in a refrigerator at 4°C.
PUM
| Property | Measurement | Unit |
|---|---|---|
| weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com