Anti-angiogenic adenovirus
A technology of recombinant adenovirus and angiostatin, which is applied in the fields of molecular biology and virology, can solve the problem of cancer being incurable
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0135] Example 1: Construction of Adenovirus Expressing Endostatin or Angiostatin
[0136] This example describes the construction of recombinant adenovirus type 5 (Ad5) expressing endostatin and / or angiostatin.
[0137] A plasmid carrying the 5' portion of the adenovirus type 5 genome sequence was modified to carry a deletion of the region of nucleotides -304 to -255 upstream of the Ela initiation site to render E1a expression cancer-selective (as previously described in U.S. Pat. No. 9,073,980). The modified plasmid is hereinafter referred to as a TAV plasmid, and any viral particles produced therefrom are hereinafter referred to as TAV adenovirus.
[0138] The TAV plasmid was further modified to carry a SalI site at the beginning of the E1b-19k region and an XhoI site 200 bp 3' to the SalI site to facilitate insertion of the therapeutic transgene. To delete the E1b-19k region 200 bp, the plasmid was cut with SalI and XhoI and self-ligated. The nucleotide sequence of the ...
Embodiment 2
[0147] Example 2: Construction of Adenovirus Expressing Endostatin and / or Angiostatin
[0148] This example describes the construction of recombinant adenovirus type 5 (Ad5) expressing endostatin and / or angiostatin.
[0149] A plasmid carrying the 5' portion of the adenovirus type 5 genome sequence was modified to carry a deletion of the region of nucleotides -304 to -255 upstream of the Ela initiation site to render E1a expression cancer-selective (as previously described in U.S. Pat. No. 9,073,980). The modified plasmid is hereinafter referred to as a TAV plasmid, and any viral particles produced therefrom are hereinafter referred to as TAV adenovirus.
[0150] The TAV plasmid was further modified to carry a SalI site at the beginning of the E1b-19k region and an XhoI site 200 bp 3' to the SalI site to facilitate insertion of the therapeutic transgene. To delete the E1b-19k region 200 bp, the plasmid was cut with SalI and XhoI and self-ligated. The nucleotide sequence of ...
Embodiment 3
[0167] Example 3: Anticancer Activity of Adenoviruses Expressing Endostatin or Angiostatin
[0168] This example describes the anticancer activity of recombinant adenoviruses expressing endostatin or angiostatin prepared as described in Example 1.
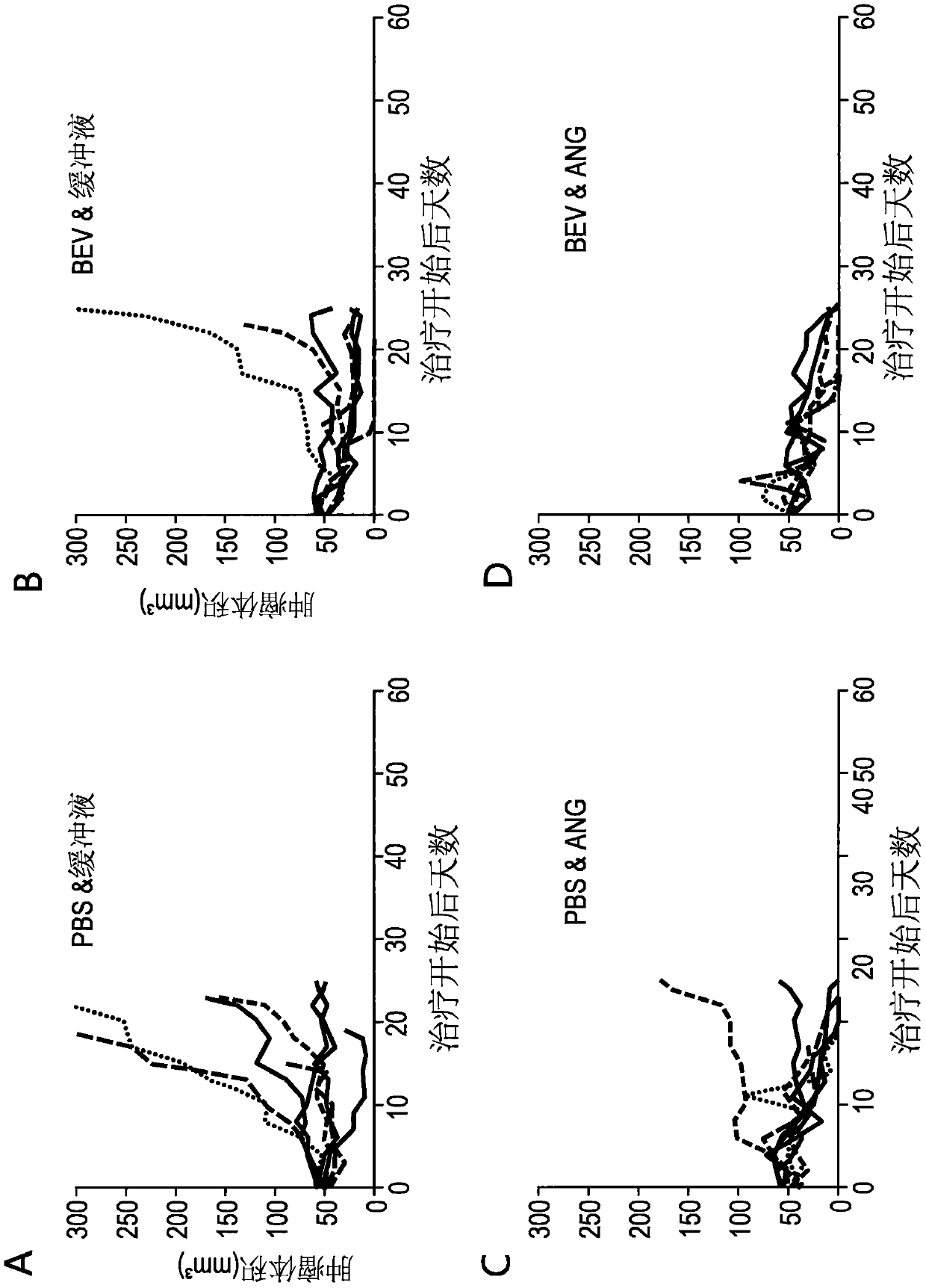
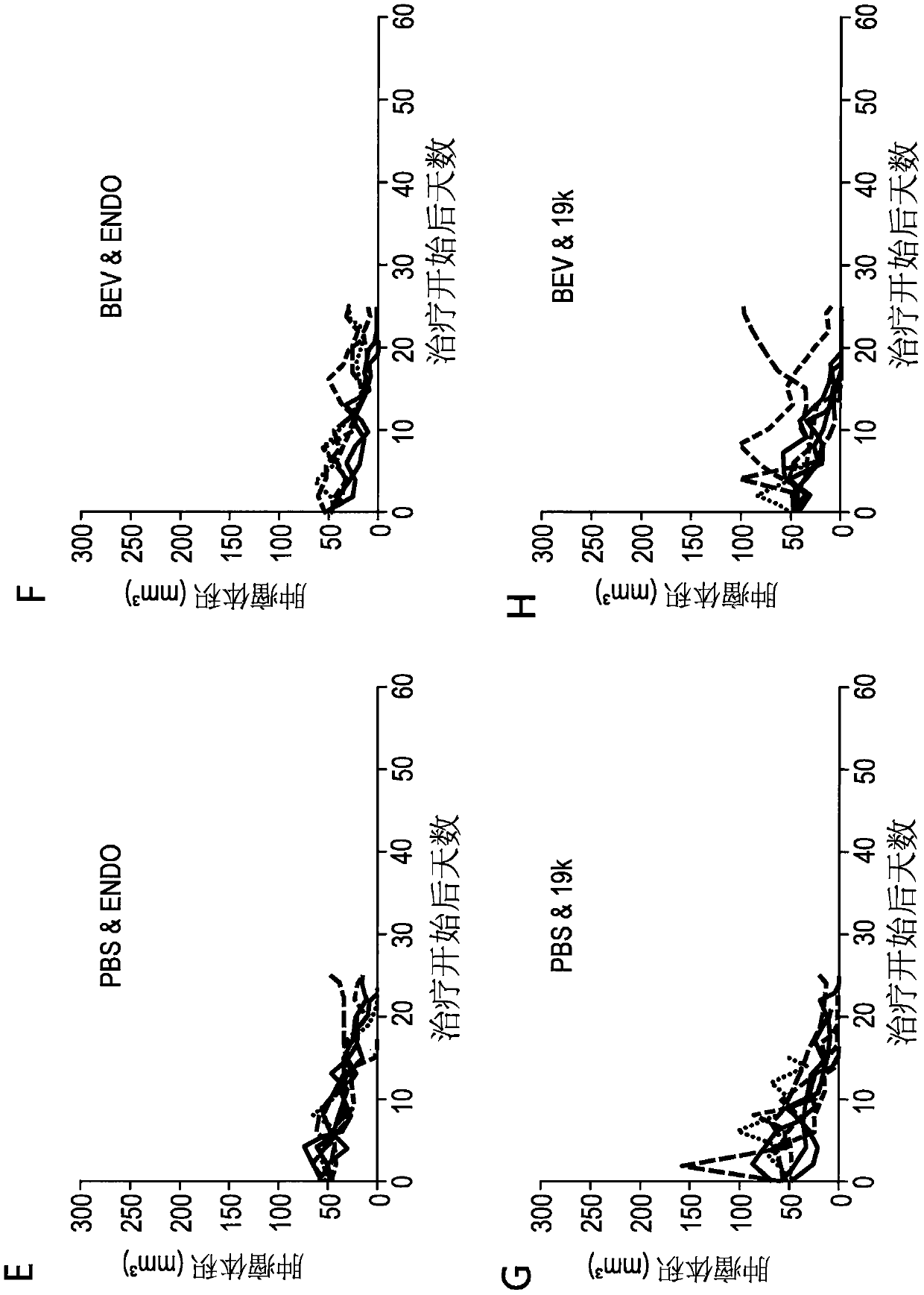
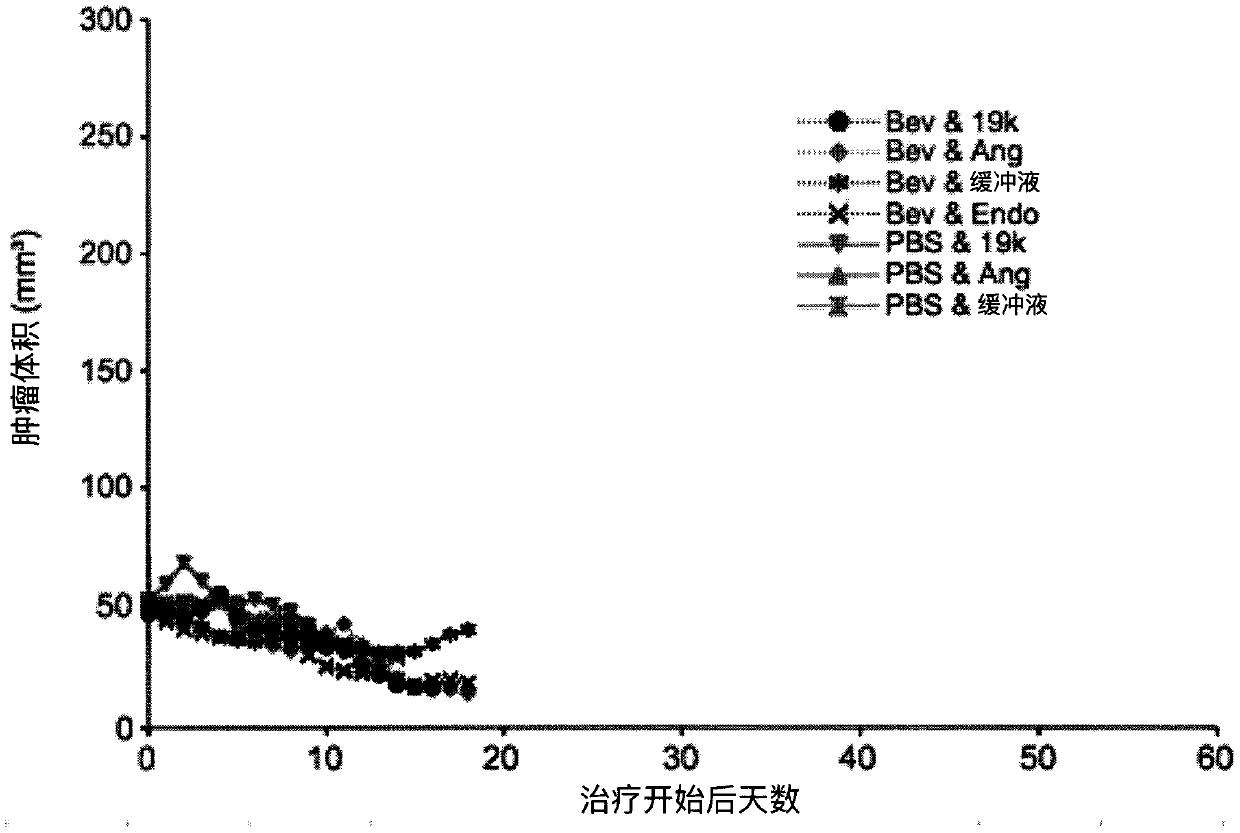
[0169] On days 0, 4 and 8, at 1x10 9 PFU / dose three intratumoral injections of buffer, TAV-Δ19k, TAV-Endo, or TAV-Ang adenovirus, and / or four intraperitoneal injections of phosphate-buffered saline (PBS) or shellfish on days 1, 5, 9, and 13 The mouse homologue of valtumab (Bev) was used to treat ADS-12 tumor-bearing 129S4 mice. Figure 1-3 Initial results, including tumor volume and progression-free survival, are depicted in . Figure 4-6 Further results after tracking mice for a longer period of time are depicted in .
[0170] These results demonstrate that endostatin- and angiostatin-expressing adenoviruses are effective in reducing tumor volume, and that endostatin- and angiostatin-expressing adenoviruses and bevacizumab act ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com