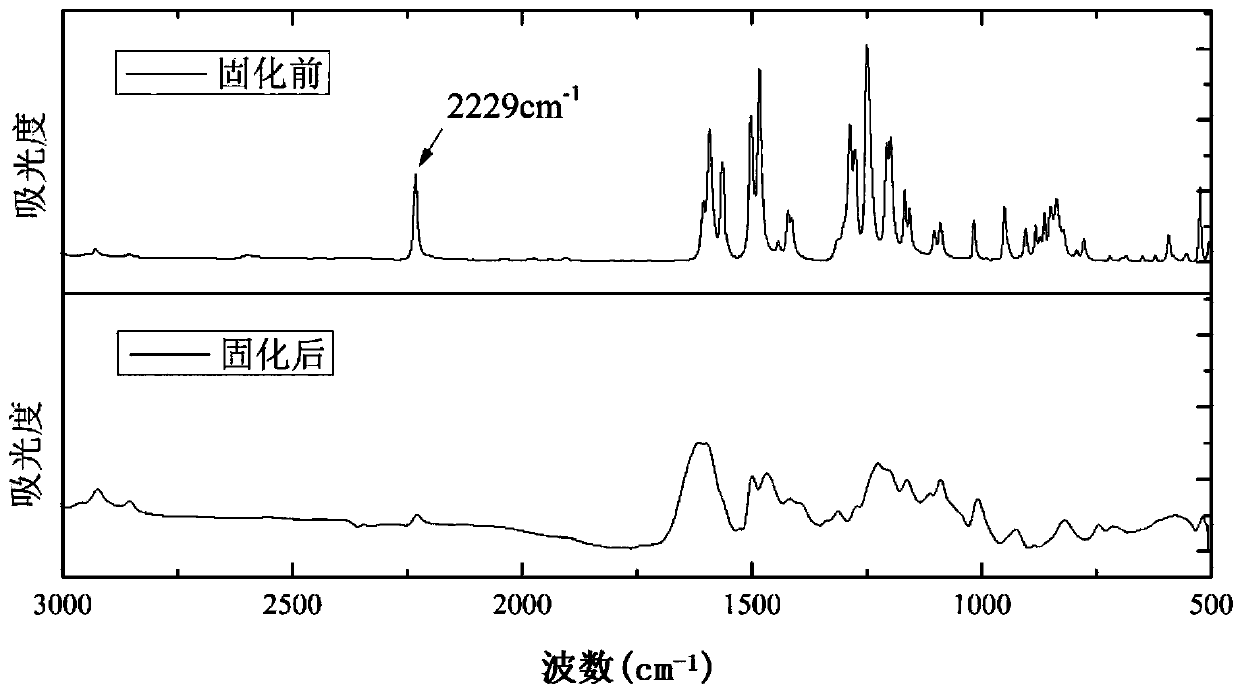
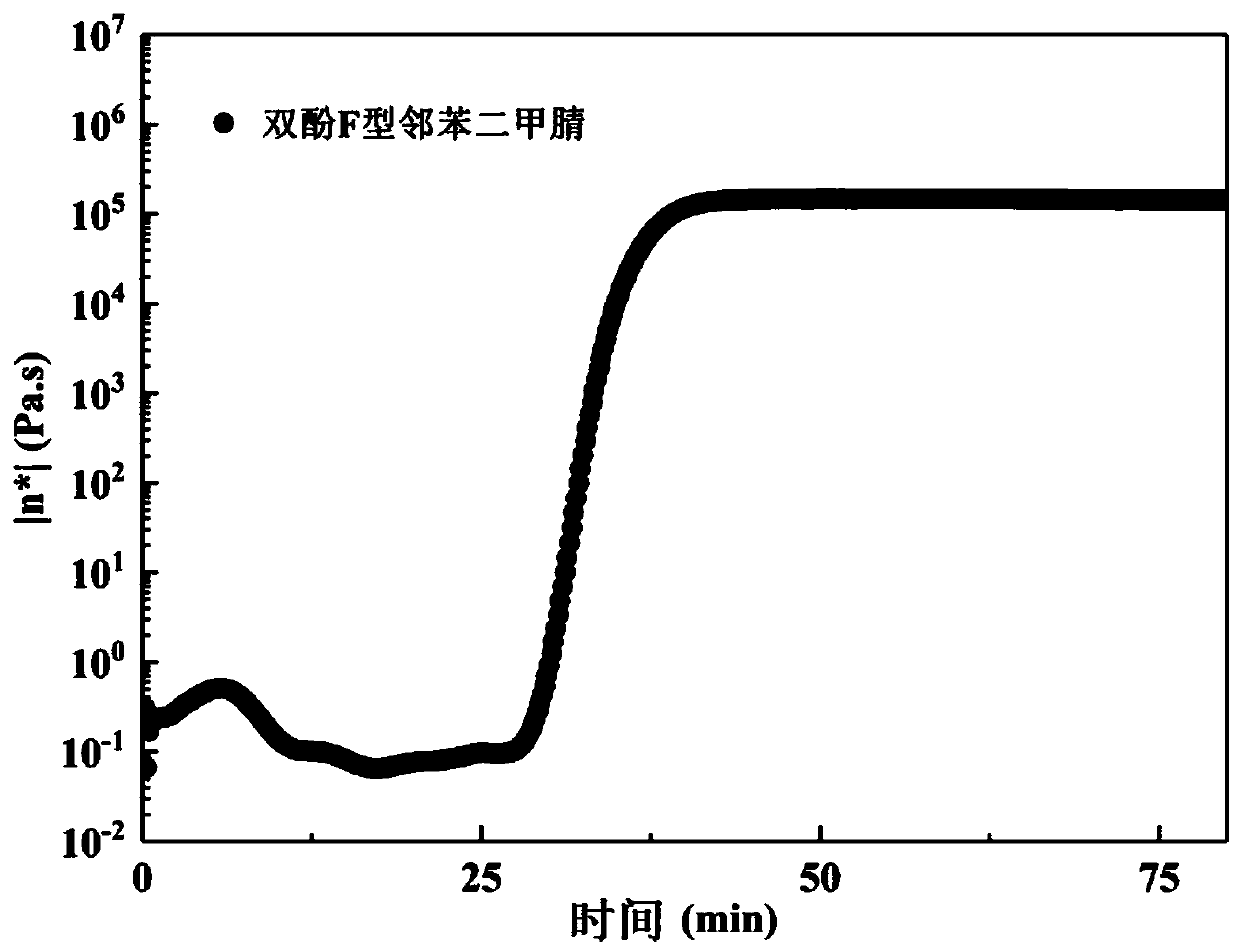
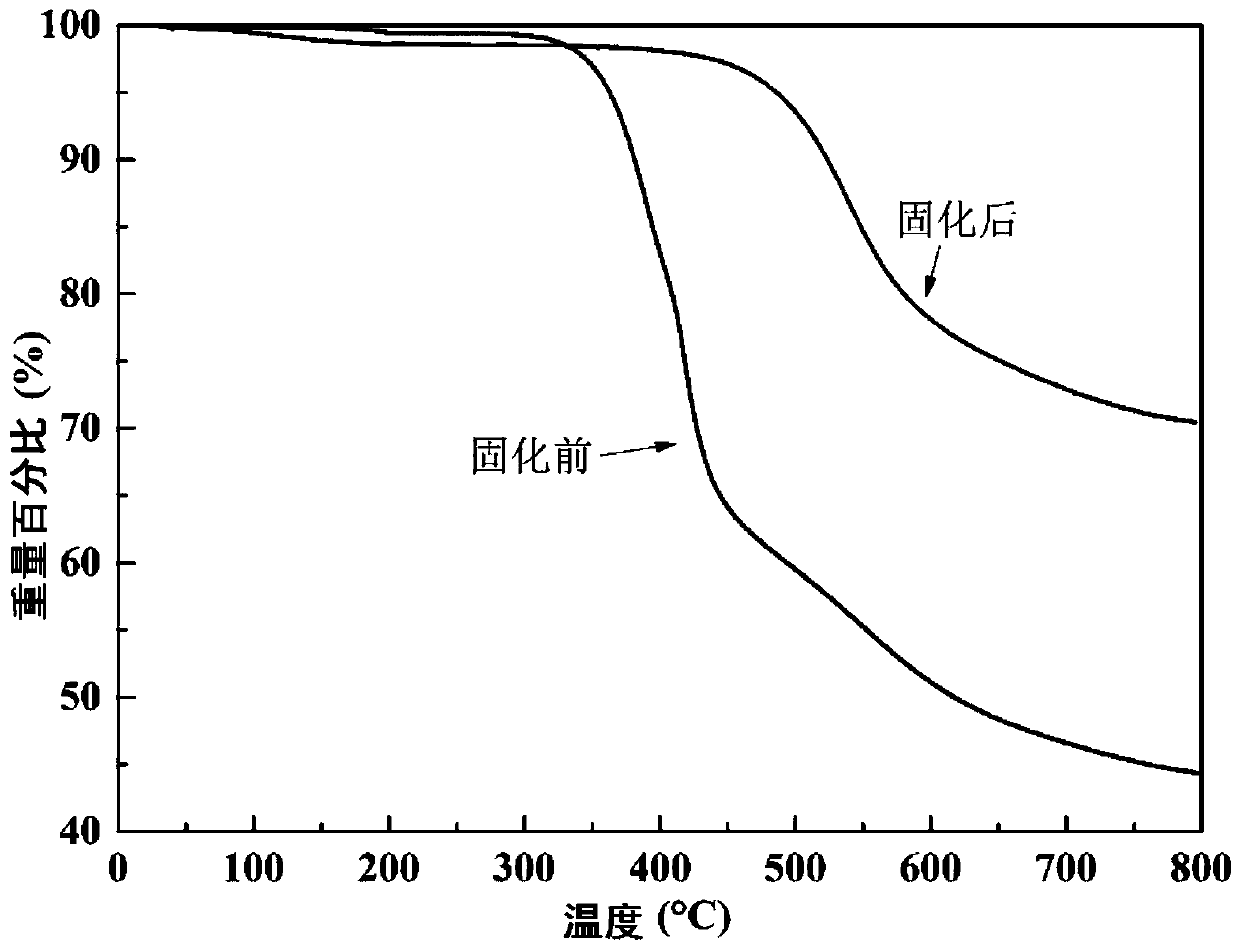
Alkyl-containing phthalonitrile resin based on autocatalytic curing and preparation method thereof
A technology of alkyl phthalonitrile resin and alkyl phthalonitrile, which is applied to the preparation of organic compounds, carboxylic acid nitrile preparation, chemical instruments and methods, etc., and can solve the problem of increasing the preparation of phthalonitrile resin Problems such as cost, limitation of wide range of applications, complex synthesis and purification
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0067] (1) Preparation of phthalonitrile monomer derived from bisphenol F
[0068] In this embodiment, bisphenol F (CAS No.: 620-92-8) and 4-nitrophthalonitrile are used as raw materials, and potassium carbonate is used as a catalyst. The bisphenol F (CAS No.: 620-92-8) , The mol ratio of 4-nitrophthalonitrile and salt of wormwood is 1.0:2.3:1.1, and the described bisphenol F consumption is 3.01g, and the structural formula of described bisphenol F is as follows:
[0069]
[0070] Dissolving bisphenol F and 4-nitrophthalonitrile in 20 mL of NMP solvent, then adding potassium carbonate to the resulting solution, and then incubating at 30°C for 12 hours to obtain a reaction solution containing a solid reaction product; The reaction solution was filtered to obtain a solid reaction product, which was then washed with deionized water and methanol (each washing solution was washed twice), and the solid reaction product was collected, and the solid reaction product was dried at 10...
Embodiment 2
[0088] (1) Preparation of phthalonitrile monomer derived from dihydric phenol
[0089] In this embodiment, dihydric phenol (CAS No.: 2081-08-05) and 4-nitrophthalonitrile are used as raw materials, and potassium carbonate is used as a catalyst. The dihydric phenol, 4-nitrophthalonitrile The mol ratio with salt of wormwood is 1.0:2.3:1.1, and described dihydric phenol consumption is 3.2g, and the structural formula of described dihydric phenol is as follows:
[0090]
[0091] Dihydric phenol and 4-nitrophthalonitrile were dissolved in 25 mL of NMP solvent, potassium carbonate was added to the resulting solution, and then reacted at 30° C. for 10 hours to obtain a reaction solution containing a solid reaction product; the obtained The reaction solution was filtered to obtain a solid reaction product, which was then washed with deionized water and methanol (each wash was washed twice), and the resulting solid reaction product was collected, and the solid reaction product was d...
Embodiment 3
[0099] (1) Preparation of phthalonitrile monomer derived from dihydric phenol
[0100] In this embodiment, dihydric phenol (CAS No.: 1576-13-2) and 4-nitrophthalonitrile are used as raw materials, and potassium carbonate is used as a catalyst. The dihydric phenol, 4-nitrophthalonitrile The mol ratio with salt of wormwood is 1.0:2.3:1.1, and described dihydric phenol consumption is 3.3g, and the structural formula of described dihydric phenol is as follows:
[0101]
[0102] Dihydric phenol and 4-nitrophthalonitrile were dissolved in 26 mL of DMSO solvent, potassium carbonate was added to the resulting solution, and then reacted at 25° C. for 15 hours to obtain a reaction solution containing a solid reaction product; the obtained The reaction solution was filtered to obtain a solid reaction product, which was then washed with deionized water and methanol (each wash was washed twice), and the resulting solid reaction product was collected, and the solid reaction product was d...
PUM
| Property | Measurement | Unit |
|---|---|---|
| storage modulus | aaaaa | aaaaa |
| carbon residual rate | aaaaa | aaaaa |
| carbon residual rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com