Preparation for producing COVID-19 antibody after oral administration and preparation method of preparation
A coronavirus, production method technology, applied in the biological field, can solve problems such as the inability to effectively prevent the second, third and other infections
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] 1. Use primer P1, such as SEQ No.1
[0021] (CGCTAGACTGTTCTTATTGTTAACACAAGGGAGAAGAGCATATGAATATCTCTCCTTAG) and primer P2
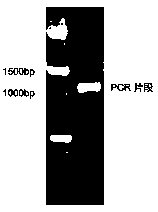
[0022] (GCAGCGACAGCGGCAGAAAATGGCGAGCAAATTTATTCAGTGTAGGCTGGAGCTGCTTC) is carried out PCR with pKD3 as template, obtains the PCR fragment that is 1091bp in length ( figure 1 ). Purification was carried out with Tiangen Biochemical Technology (Beijing) Co., Ltd. General Product Purification Kit (Product No. DP204), and the DNA concentration was measured with a NanoDrop2000 spectrophotometer.
[0023] Wild-type Salmonella typhimurium (Salmonella typhimurium, LT2) with pKD46 plasmid was cultivated in 1 mL of LB. Culture medium plus ampicillin with a final concentration of 50 μg / mL was cultured overnight at 30° C. on a shaker at 150 r / min. The next day, the overnight culture product was inoculated 1:100 in 50 mL of SOB medium with a final concentration of 10 mM arabinose and 50 μg / mL of ampicillin, and then cultured on a shaker at 30 °C at 150 r / min for...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com