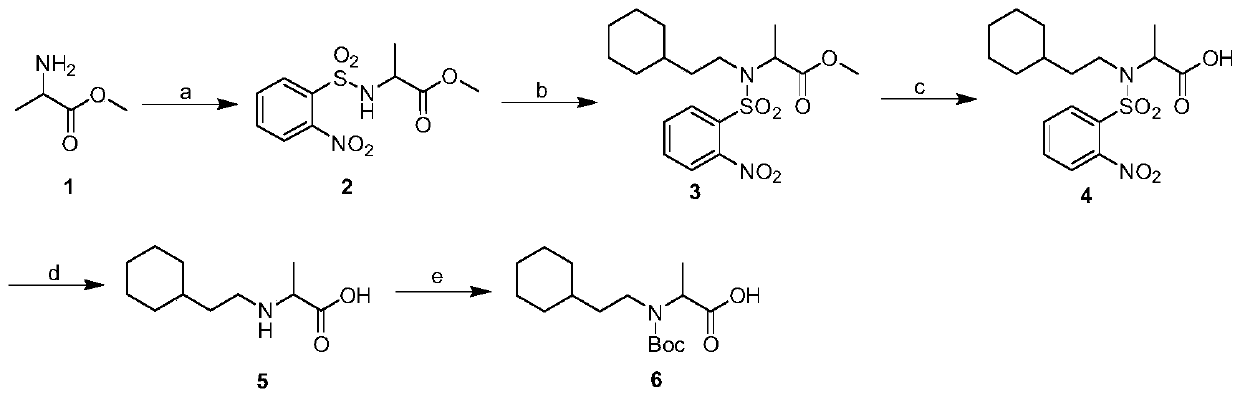
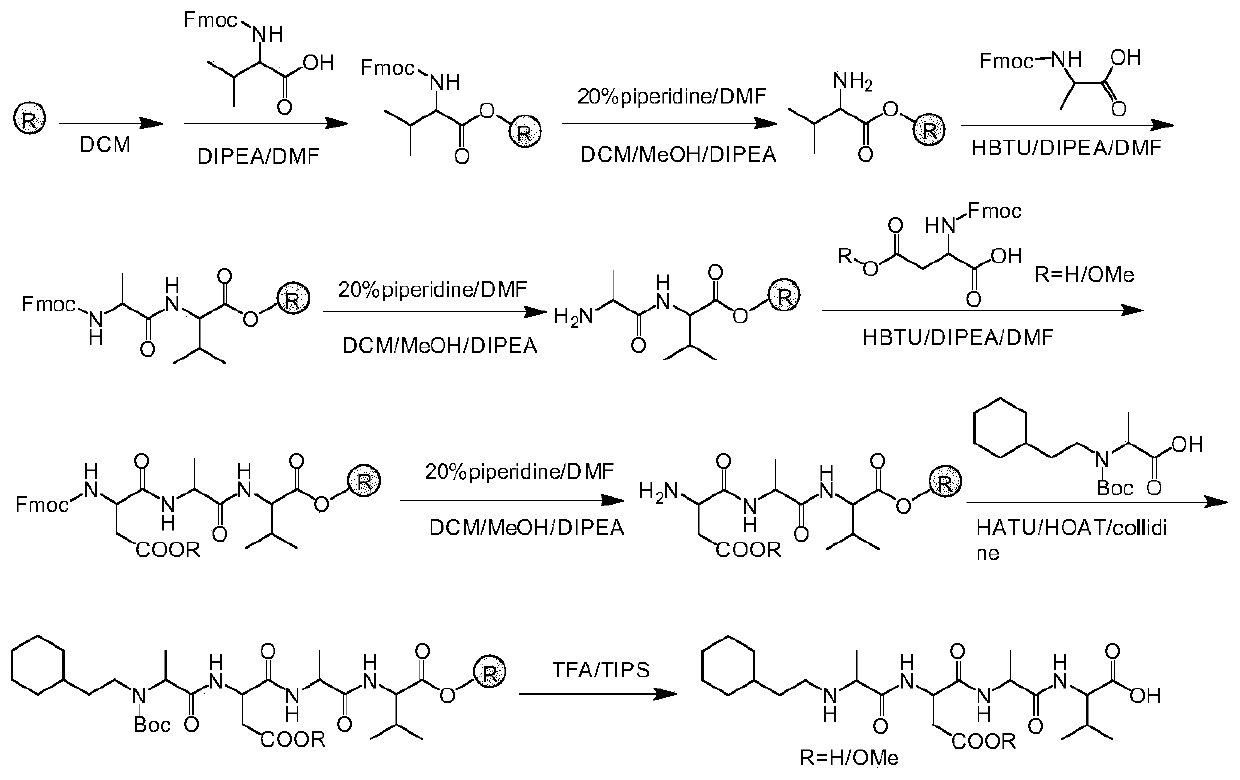
Polypeptide with nNOS-Capon uncoupling activity and application of polypeptide
A technology of uncoupling and che-gly-asp, which is applied in the field of polypeptides with nNOS-Capon uncoupling activity, can solve the problem that NMDAR antagonists cannot develop therapeutic drugs, and achieve obvious neuroprotective effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] Example 1 N-Cyclohexylethyl-Ala-Glu-Ala-Val (Che-AEAV)
[0050] After activating the dry 2-chlorotrimethyl chloride resin (loading rate 1.056mmol), use Fmoc-Val-OH as the starting material, repeat the Fmoc removal and amino acid condensation reaction many times, the specific synthetic route is as follows figure 2 As shown, 155.5 mg of Che-AEAV was obtained with a yield of 15.61%. HPLC:t R =13.45min.MS(ESI)calcd for C 24 h 42 N 4 o 7 [M-H] - :497.3; found: m / z 497.3. 1 H NMR (300MHz, DMSO-d 6 )δ12.30(s, 2H), 8.64(d, J=8.3Hz, 1H), 8.07(d, J=7.1Hz, 1H), 7.90(d, J=8.6Hz, 1H), 4.34(t, J=7.0Hz, 2H), 4.10(dd, J=8.5, 5.7Hz, 1H), 3.89(s, 1H), 2.78(s, 3H), 2.24(t, J=8.0Hz, 2H), 2.01– 1.89(m,1H),1.87(d,J=5.2Hz,1H),1.83–1.68(m,1H),1.61–1.47(m,5H),1.42(t,J=7.6Hz,2H),1.33 (d,J=6.8Hz,3H),1.18(d,J=7.0Hz,6H),0.84(d,J=6.8Hz,9H).
Embodiment 2
[0051] Example 2 N-Cyclohexylethyl-Ala-Glu-Trp-Val (Che-AEWV)
[0052] According to the synthesis method in Example 1, 76.3 mg of Che-AEWV was obtained with a yield of 12.45%. HPLC:t R =14.15min.MS(ESI)calcd for C 32 h 47 N 5 o 7 [M-H] - :612.3; found: m / z 612.4. 1 H NMR (300MHz, DMSO-d 6 )δ13.04–11.60(m,2H),10.75(s,1H),8.58(d,J=8.5Hz,1H),8.08(t,J=8.5Hz,2H),7.82–6.38(m,5H ), 4.65(d, J=4.9Hz, 1H), 4.33(d, J=5.4Hz, 1H), 4.21–4.06(m, 1H), 3.81(d, J=7.0Hz, 1H), 3.09(d ,J=10.0Hz,2H),2.97–2.63(m,3H),2.15–2.05(m,3H),2.06(s,1H),1.87(s,1H),1.73(d,J=7.9Hz, 1H), 1.60(d, J=9.5Hz, 5H), 1.55–1.32(m, 3H), 1.23–1.12(m, 4H), 1.13(s, 3H), 0.87–0.71(m, 6H).
Embodiment 3
[0053] Example 3 N-Cyclohexylethyl-Ala-Asp-Trp-Val (Che-ADWV)
[0054] According to the synthesis method in Example 1, 38.4 mg of Che-ADWV was obtained with a yield of 6.41%. HPLC:t R =14.35min.MS(ESI)calcd for C 31 h 45 N 5 o 7 [M-H] - :598.3; found: m / z 598.3. 1 H NMR (300MHz, DMSO-d 6 )δ10.75(s,2H),8.66(s,1H),7.96(s,2H),7.55(d,J=7.8Hz,1H),7.28(d,J=8.4Hz,1H),7.13– 6.77(m,4H),4.58(s,2H),4.12(s,1H),3.68(s,1H),3.08(s,2H),2.93(s,2H),2.67–2.35(m,3H) ,2.01(s,1H),1.58(s,5H),1.38(d,J=7.5Hz,3H),1.23(d,J=6.8Hz,5H),1.12–0.99(m,3H),0.84( d,J=4.1Hz,6H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com