A controllable synthesis method of highly stereoregular polymethyl methacrylate
A technology of polymethyl methacrylate and methyl methacrylate, which is applied in the field of polymer science, can solve the problems of difficult control of chain transfer and side reactions, high reactivity and low stability of growth groups, and achieves a synthetic route. Clear and feasible effects, improved tacticity, and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0023] The present invention will be further described below in conjunction with the accompanying drawings.
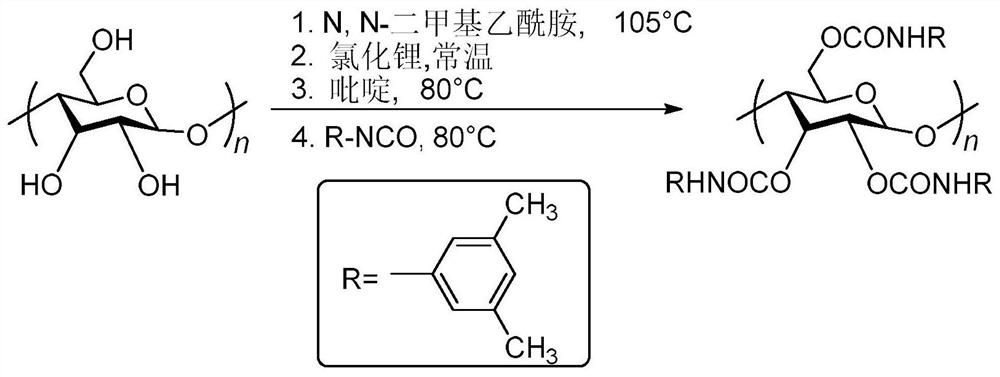
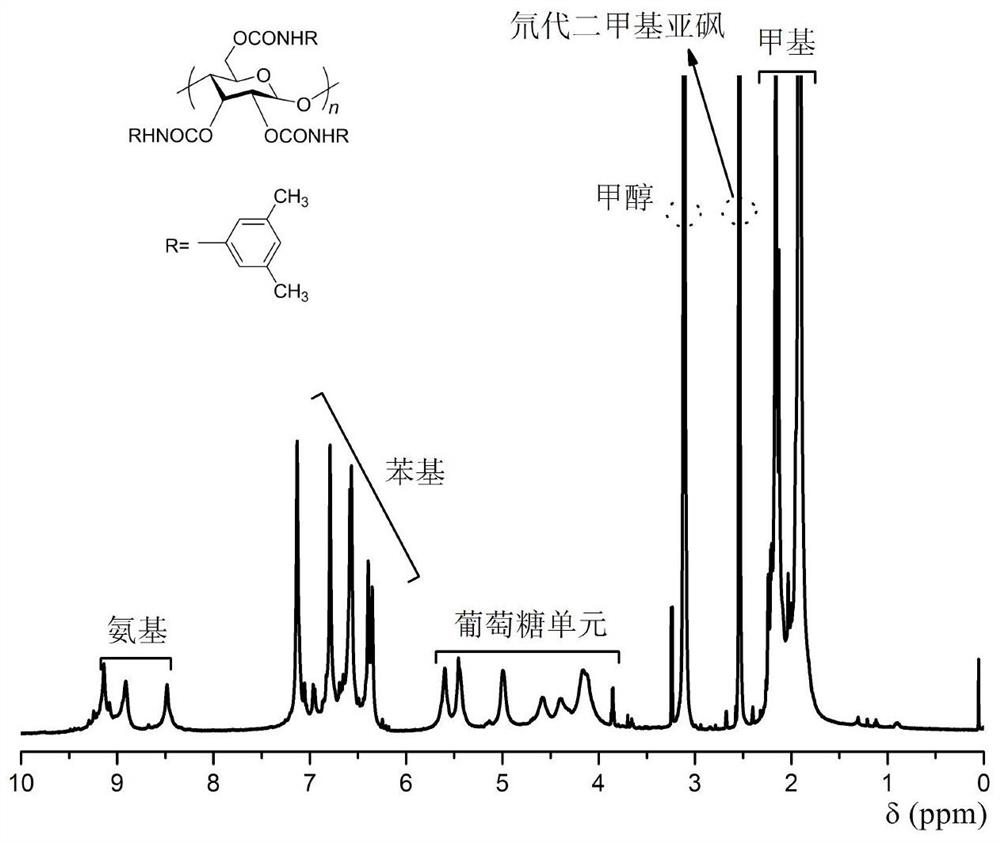
[0024] It is proposed to use amylose derivatives with regular helical structure as additives to induce methyl methacrylate to form highly stereoregular polymers through free radical polymerization, and to further explore the effect of amylose derivatives on polymethyl methacrylate. Effect of ester tacticity. In the present invention, firstly, amylose is used as matrix raw material, and 3,5-dimethylphenyl isocyanate is used as derivatization reagent, and the helical polymer amylose-tris(3,5-dimethylbenzene) is synthesized by traditional esterification method. base carbamate) (ADMPC). On this basis, the synthesized amylose derivative was added as an additive to the radical polymerization reaction of methyl methacrylate, and its regular helical structure was used to induce the radical polymerization behavior of methyl methacrylate, and the final preparation Polymethyl m...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com