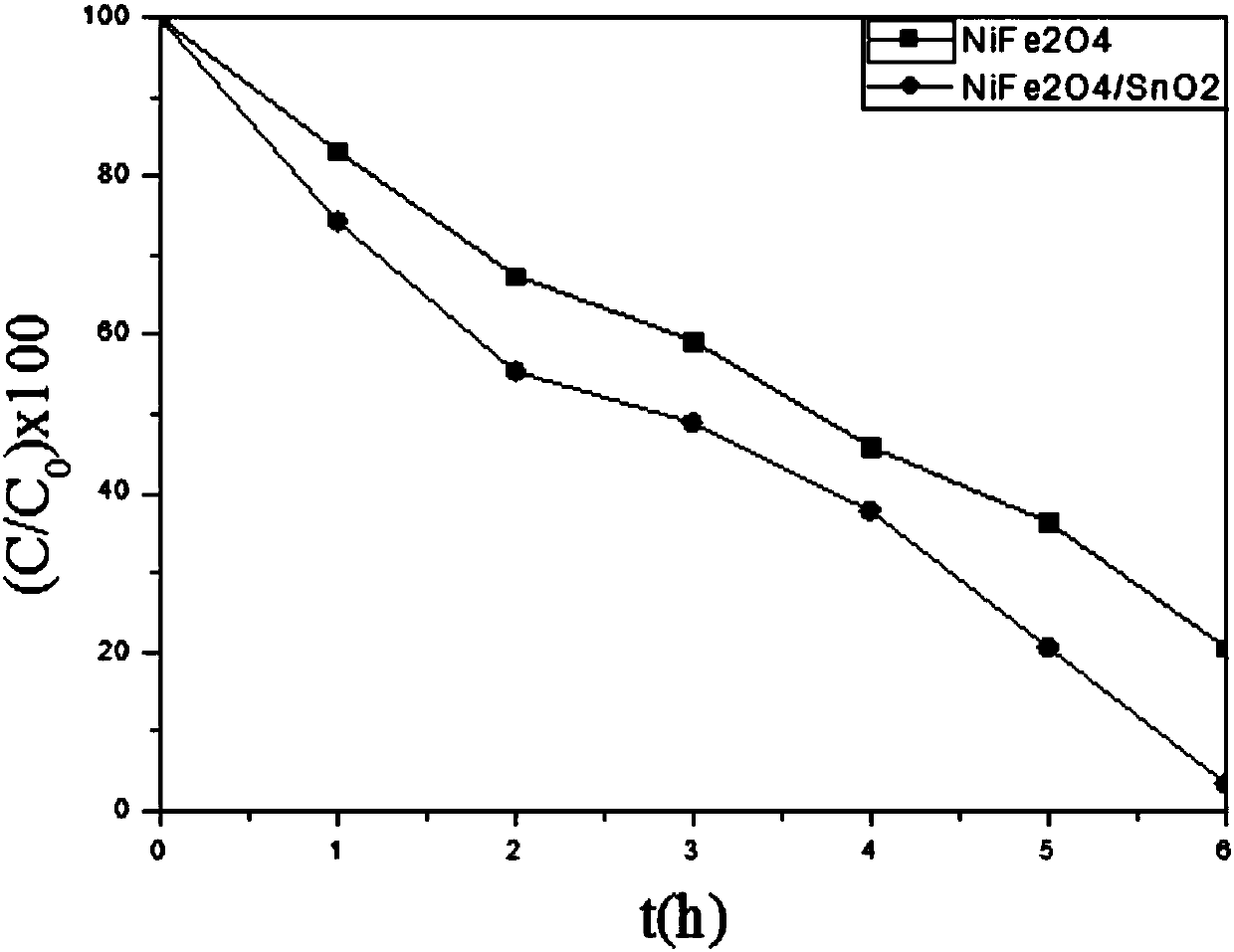
A kind of preparation method of nife2o4/sno2 composite photocatalyst
A catalyst and composite light technology, applied in the field of nanotechnology, can solve problems such as poor shape and poor stability, and achieve the effects of simple production equipment, simple synthesis process, and short synthesis cycle
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
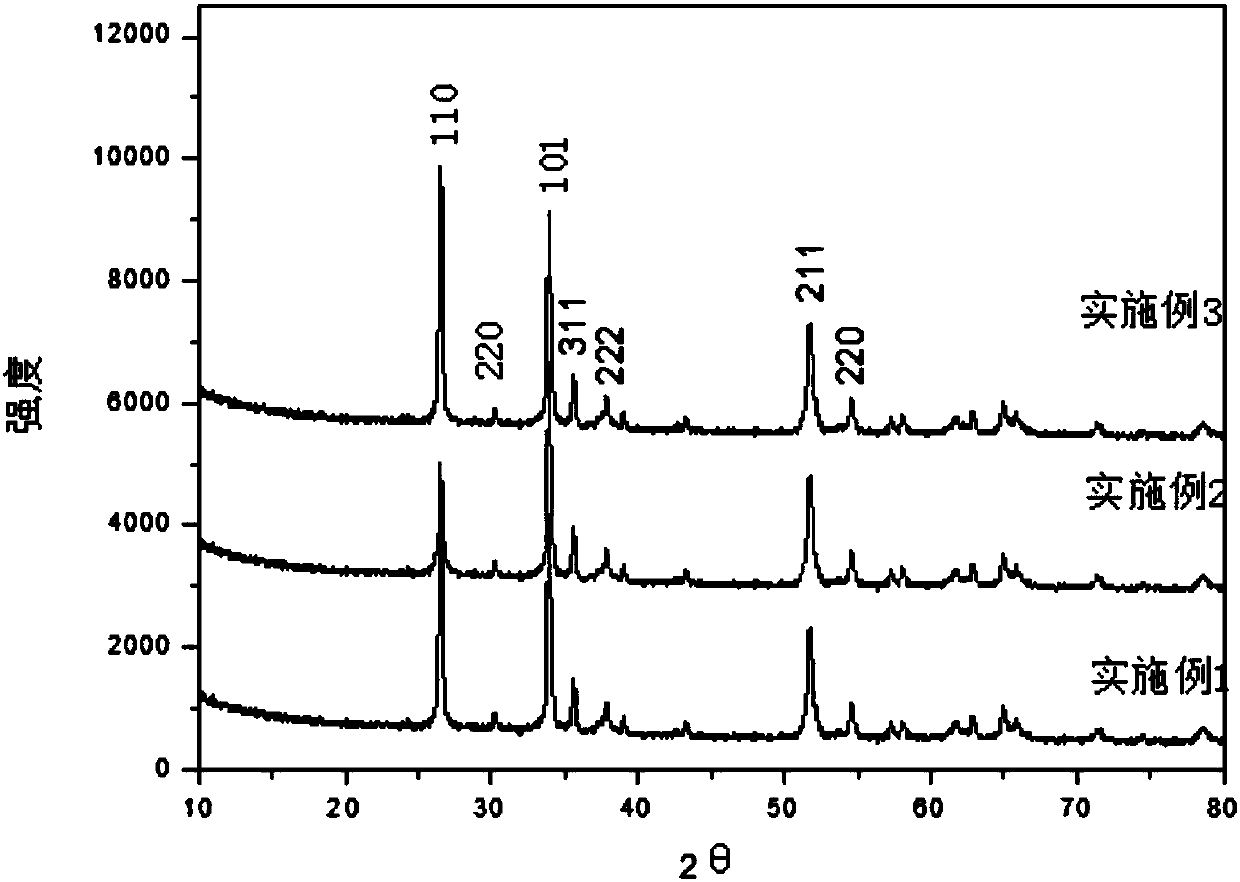
Embodiment 1
[0028] (1) 0.14g of SnCl 4 ·5H 2 O and 0.48g NaOH were dissolved in 50ml deionized water, then 0.02gNiFe 2 o 4 Nano powder, NiFe 2 o 4 The concentration of the nanopowder is 0.002mol / L, and then mixed under stirring conditions;
[0029] The above-mentioned NiFe2O4 nanopowder and SnCl 4 ·5H 2 The consumption of O is calculated according to the ratio of 1:4 by the molar ratio of Ni:Sn;
[0030] The NiFe 2 o 4 Nano powder is NiFe 2 o 4 nano octahedron;
[0031] (2) Place the mixed material obtained after mixing in step (1) in a hydrothermal kettle, and control the temperature to 220° C. to react for 8 hours to obtain a reaction product;
[0032](3) After the reaction product is naturally cooled, it is centrifugally filtered, and the obtained product is washed 3-5 times with deionized water, then washed twice with absolute ethanol, and then dried at a temperature of 60°C for 12 hours to obtain the NiFe 2 o 4 SnO grows on the surface of nanopowder 2 Pale yellow nanop...
Embodiment 2
[0037] (1) 0.105g of SnCl 4 ·5H 2 O and 0.48g NaOH were dissolved in 70ml deionized water, then 0.03gNiFe 2 o 4 Nano powder, NiFe 2 o 4 The concentration of the nanopowder is 0.002mol / L, and then mixed under stirring conditions;
[0038] The above NiFe 2 o 4 Nanopowder and SnCl 4 ·5H 2 The consumption of O is calculated according to the ratio of 1:2 by the molar ratio of Ni:Sn;
[0039] The NiFe 2 o 4 Nano powder is NiFe 2 o 4 nano octahedron;
[0040] (2) Place the mixed material obtained after mixing in step (1) in a hydrothermal kettle, and control the temperature to 220° C. to react for 8 hours to obtain a reaction product;
[0041] (3) After the reaction product is naturally cooled, it is centrifugally filtered, and the obtained product is washed 3-5 times with deionized water, then washed twice with absolute ethanol, and then dried at a temperature of 60°C for 12 hours to obtain the NiFe 2 o 4 SnO grows on the surface of nanopowder 2 Pale yellow nanopowd...
Embodiment 3
[0043] (1) 0.14g of SnCl 4 ·5H 2 O and 0.48g NaOH were dissolved in 60ml deionized water, then 0.02NiFe 2 o 4 Nano powder, NiFe 2 o 4 The concentration of the nanopowder is 0.002mol / L, and then mixed under stirring conditions;
[0044] The above NiFe 2 o 4 Nanopowder and SnCl 4 ·5H 2 The consumption of O is calculated according to the ratio of 1:4 by the molar ratio of Ni:Sn;
[0045] The NiFe 2 o 4 Nano powder is NiFe 2 o 4 nano octahedron;
[0046] (2) Place the mixed material obtained in the step (1) into a hydrothermal kettle, and control the temperature to 200° C. to react for 3 hours to obtain a reaction product;
[0047] (3) After the reaction product is naturally cooled, it is centrifugally filtered, and the obtained product is washed 3-5 times with deionized water, then washed twice with absolute ethanol, and then dried at a temperature of 60°C for 12 hours to obtain the NiFe 2 o 4 SnO grows on the surface of nanopowder 2 Pale yellow nanopowder, namel...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| Mohs hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com