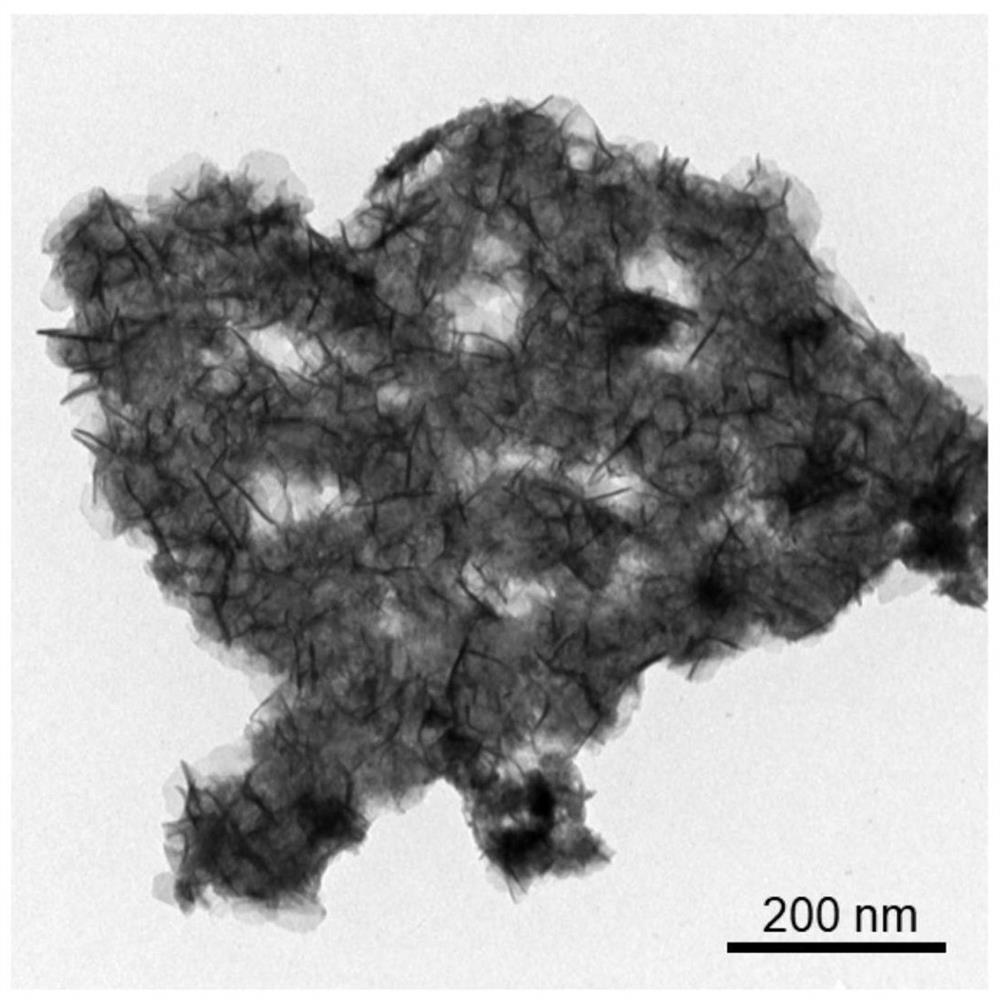
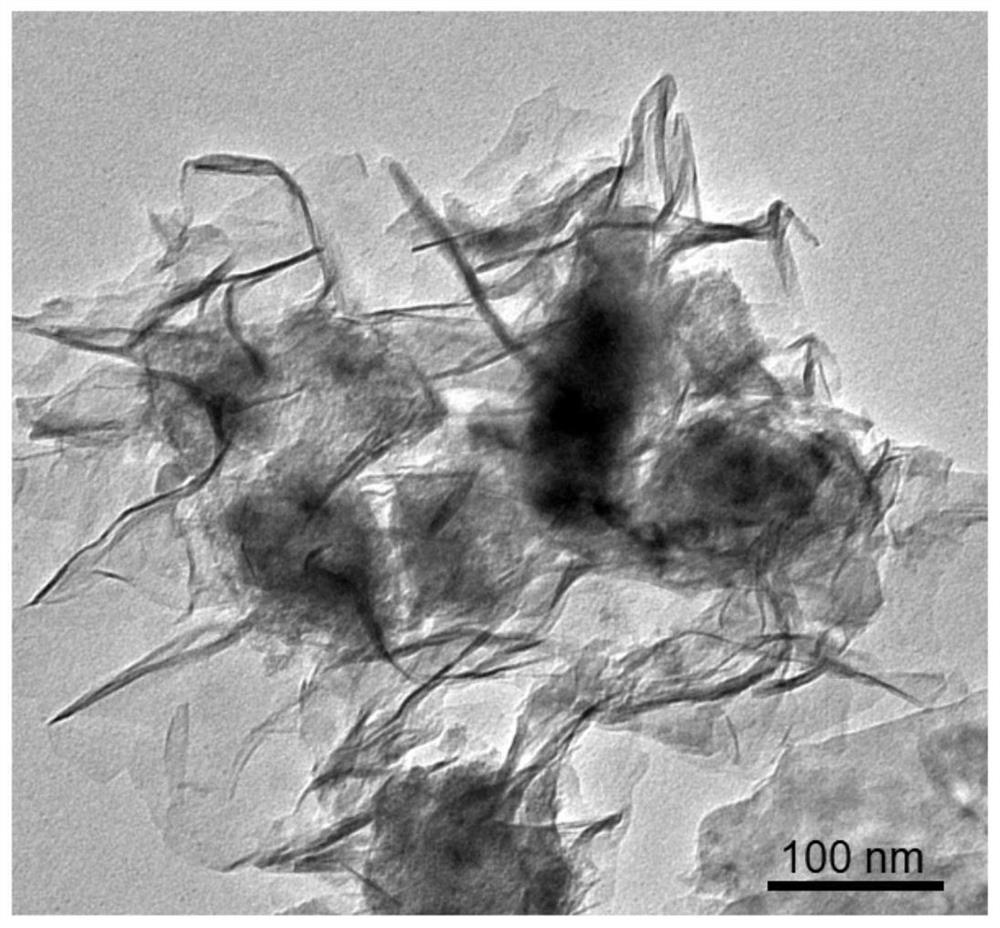
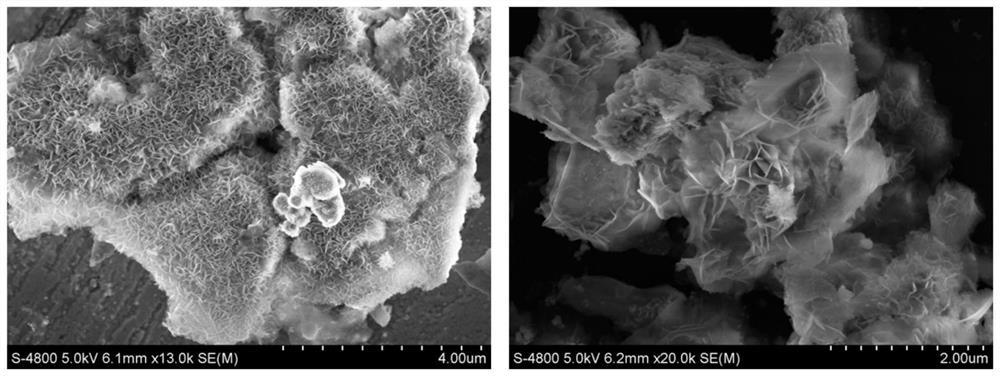
A preparation method of n,p-codoped three-dimensional co nanoflowers and the resulting materials and applications
A nanoflower, co-doping technology, applied in chemical instruments and methods, physical/chemical process catalysts, electrolysis components, etc., can solve the problems of complex process, high cost, unsatisfactory catalytic activity and stability, etc., and achieve the preparation process. The mechanism is clear, the catalytic activity is improved, and the effect of suppressing the Ostwald ripening effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] A preparation method of N, P-co-doped three-dimensional Co nanoflowers, comprising the following steps:
[0033] 1) Recrystallization of precursor and alkali metal inorganic salt saturated solution: Measure 10mL saturated NaCl solution, add 10mL0.05mol L -1 CoCl 2 Precursor solution and 5mL 0.05mol L -1 NaH 2 PO 2 Solution, oscillate to mix evenly, disperse into a watch glass and recrystallize at room temperature.
[0034] 2) Preparation of N,P-co-doped three-dimensional Co nanoflowers: the recrystallized product obtained in step 1) was heated in an ammonia atmosphere at 5°C min -1 The temperature was raised to 600° C. for heat treatment, and kept at this temperature for 3 hours. After cooling to room temperature, the final product was obtained by centrifuging and washing with water for several times.
Embodiment 2
[0036] A preparation method of N, P-co-doped three-dimensional Co nanoflowers, comprising the following steps:
[0037] 1) Recrystallization of precursor and alkali metal inorganic salt saturated solution: measure 10mL saturated Na 2 SO 4 solution, add 10mL0.05mol L -1 CoCl 2 Precursor solution and 5mL 0.05mol L -1 NaH 2 PO 2 Solution, oscillate to mix evenly, disperse into a watch glass and recrystallize at room temperature.
[0038] 2) Preparation of N,P-co-doped three-dimensional Co nanoflowers: the recrystallized product obtained in step 1) was heated in an ammonia atmosphere at 5°C min -1 The temperature was raised to 600° C. for heat treatment, and kept at this temperature for 3 hours. After cooling to room temperature, the final product was obtained by centrifuging and washing with water for several times.
Embodiment 3
[0040] A preparation method of N, P-co-doped three-dimensional Co nanoflowers, comprising the following steps:
[0041] 1) Recrystallization of precursor and alkali metal inorganic salt saturated solution: measure 10mL saturated MgCl 2 solution, add 10mL0.05mol L -1 CoCl 2 Precursor solution and 5mL 0.05mol L -1 NaH 2 PO 2 Solution, oscillate to mix evenly, disperse into a watch glass and recrystallize at room temperature.
[0042] 2) Preparation of N,P-co-doped three-dimensional Co nanoflowers: the recrystallized product obtained in step 1) was heated in an ammonia atmosphere at 5°C min -1 The temperature was raised to 600° C. for heat treatment, and kept at this temperature for 3 hours. After cooling to room temperature, the final product was obtained by centrifuging and washing with water for several times.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com