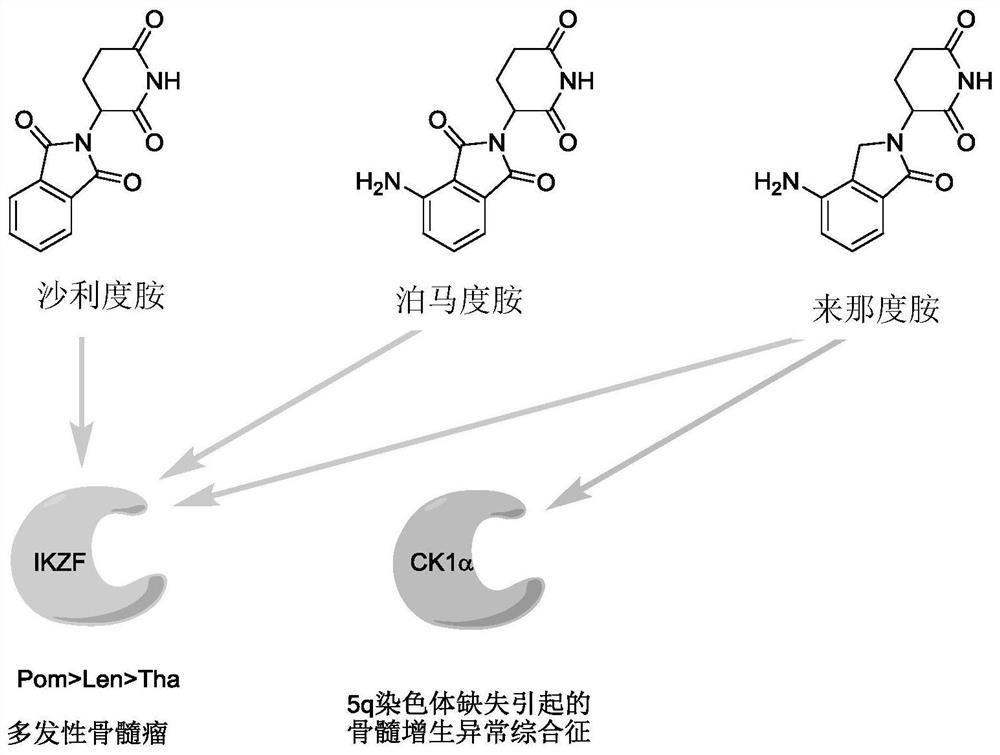
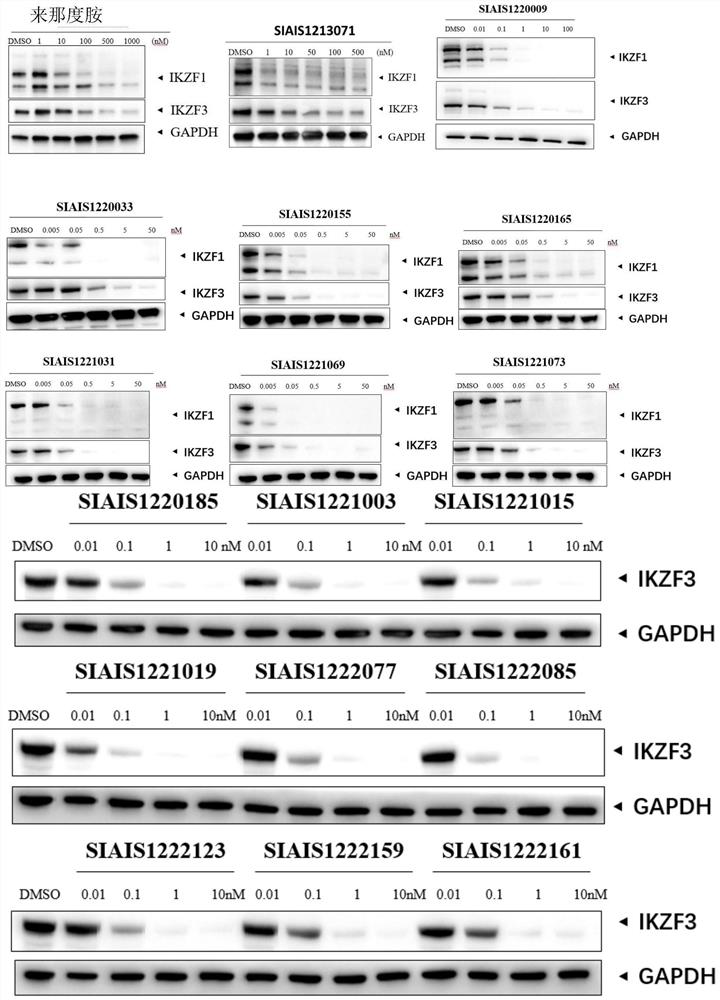
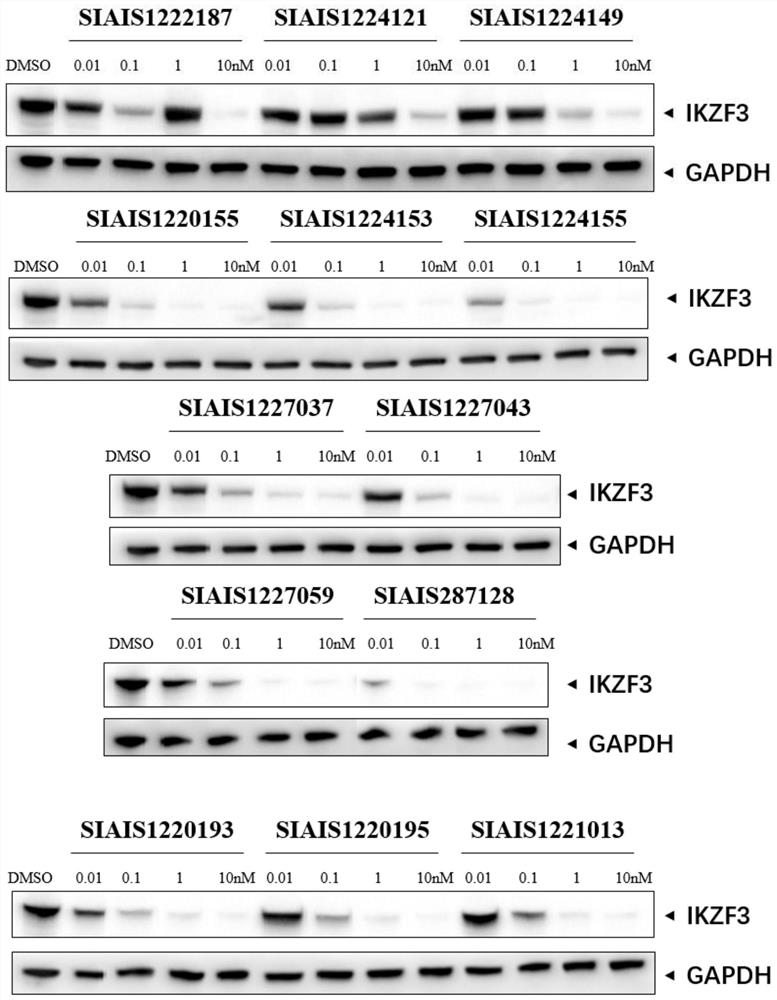
Sulfur-containing compound based on glutaryl imide skeleton and application of compound
A technology of compounds and mixtures, which is applied to sulfur-containing compounds based on glutarimide skeleton and its application field, and can solve the problems that biological activity needs to be further improved
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach 1
[0049] Therefore, one aspect of the present invention provides embodiment 1): a compound of formula (I) or a salt, solvate, isotopically enriched analog, tautomer, polymorph, stereoisomer (including Enantiomers), or mixtures of stereoisomers:
[0050]
[0051] in:
[0052] A means CH 2 or C(O);
[0053] B, U, V, W are the same or different and independently represent CH or N, wherein B, U, V and W are not N at the same time;
[0054] Y means O or S;
[0055] R means SH, and L and X 1 does not exist; or
[0056] R represents S, L represents optionally substituted linear or branched chain alkyl, and X 1 does not exist; or
[0057] R stands for S(O) or S(O) 2 , L represents an optionally substituted linear or branched chain alkyl or amino group, and X 1 does not exist; or
[0058] R stands for S, S(O) or S(O) 2 ,
[0059] L represents an optionally substituted linear or branched alkylene group, wherein the linear or branched alkylene group is optionally interrupted...
Embodiment approach 2
[0074]Embodiment 2): It relates to the compound of formula (I) or its salt, solvate, isotopically enriched analog, tautomer, polymorph, stereoisomer or stereoisomer as described in Embodiment 1). A mixture of isomers, wherein the ring atoms B, U, V, and W in formula (I) are the same and all are CH.
Embodiment approach 3
[0075] Embodiment 3): It relates to the compound of formula (I) or its salt, solvate, isotopically enriched analog, tautomer, polymorph, stereoisomer or stereoisomer as described in Embodiment 1). A mixture of isomers, wherein one of the ring atoms B, U, V, W in the formula (I) is N, and the rest are CH.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com