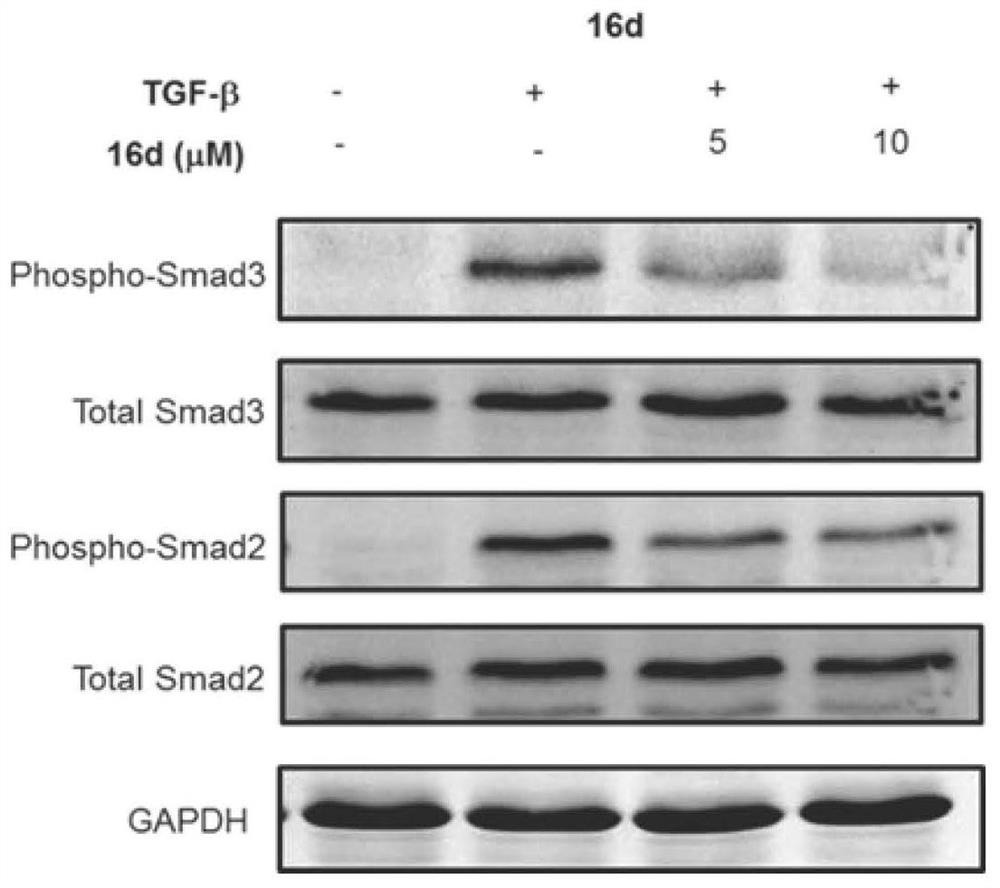
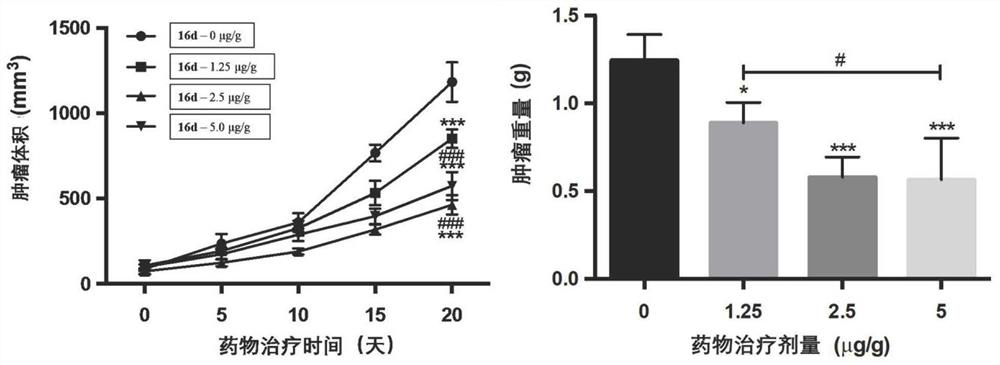
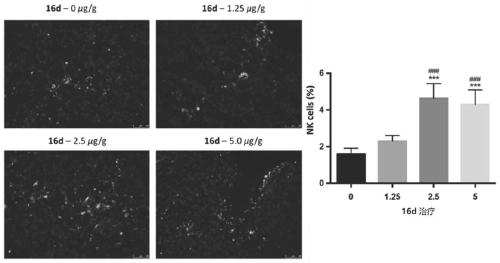
Azaindole compound and application thereof
A technology of azaindole and compounds, applied in the field of medicinal chemistry, can solve problems such as non-existence and side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0096] Embodiment 1: the synthesis of compound 5a-5j
[0097]
[0098] Synthesis of Intermediate 4a:
[0099]
[0100] Put NaH (192mg, 4.8mmol) in the reaction flask, replace nitrogen, add 7mL of anhydrous DMF to dissolve, add methyl phosphonoacetate diethyl phosphonoacetate under the condition of ice-water bath, stir for 0.5 hours, add compound 3a dropwise ( 636mg, 3.973mmol) in 7.5mL DMF solution, stirred at room temperature for 8 hours. Sampling point plate, the reaction is basically completed, quenched the reaction with water, stirred for 2 hours, extracted three times with ethyl acetate, washed with saturated brine, dried and filtered, concentrated and purified by silica gel column to obtain yellow solid compound n1 (520 mg, 60.5%); compound n1 (520mg, 2.4mmol) dissolved in THF / MeOH / H 2 O (5mL / 8mL / 8mL) mixed solution was added NaOH (288mg, 7.2mmol) and stirred at room temperature for 4 hours. Sampling point plate, the reaction is basically completed. HCl (1M) wa...
Embodiment 2
[0166] Embodiment 2: the synthesis of compound 9
[0167]
[0168] Compound 6 (208mg, 1mmol) and AlCl 3 (667mg, 5mmol) was placed in a reaction flask, replaced with nitrogen, dissolved in 20mL of DCM, and stirred at room temperature for 1 hour. Add methyl 2-chloro-2-oxoacetate (122mg, 1mmol), stir at 0°C for 0.5 hours, then warm to room temperature, and 2 Stir overnight under protective conditions. Sampling point plate, the reaction is basically completed, extraction, DCM extraction three times, combined organic phase, dried, filtered, concentrated, purified by silica gel column chromatography to obtain yellow solid compound 7 (270 mg, 92%); 1 H NMR (400MHz, CDCl 3 )δ8.71(d, J=7.8Hz, 1H), 8.49(d, J=4.7Hz, 1H), 7.63–7.53(m, 3H), 7.48(d, J=5.7Hz, 2H), 7.35( dd,J=7.8,4.8Hz,1H),3.74(s,3H),3.24(s,3H). 13 CNMR (100MHz, CDCl 3 )δ177.5, 162.6, 149.1, 144.0, 137.7, 132.1, 119.8, 119.2, 112.7, 52.9.
[0169] Put compound 7 (270mg, 0.92mmol) and LiOH (66mg, 2.76mmol) in a react...
Embodiment 3
[0171] Embodiment 3: the synthesis of compound 12
[0172]
[0173] Put NaH (112mg, 2.8mmol) in the reaction flask, replace nitrogen, add 5mL of anhydrous DMF to dissolve, add methyl phosphonoacetate diethyl phosphonoacetate (555mg, 2.2mmol) under the condition of ice-water bath, stir for 0.5 hours, A DMF solution of compound 10 (451 mg, 2.0 mmol) was added dropwise and stirred at room temperature for 8 hours. Sampling and spotting the plate, the reaction was basically completed, quenched the reaction with water, stirred for 2 hours, extracted three times with ethyl acetate, washed with saturated brine, dried, filtered, concentrated, and purified on a silica gel column to obtain yellow intermediate n7 (542 mg, 84%).
[0174] Intermediate n7 (542 mg, 1.68 mmol) was dissolved in CH 2 Cl 2 (9mL) and TFA (2mL) in a mixed solution, stirred overnight at room temperature, sampled and plated, the reaction was basically completed, and compound 11 (550mg, crude yield) was obtained....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com