Preparation method of spiropyrane photochromic material
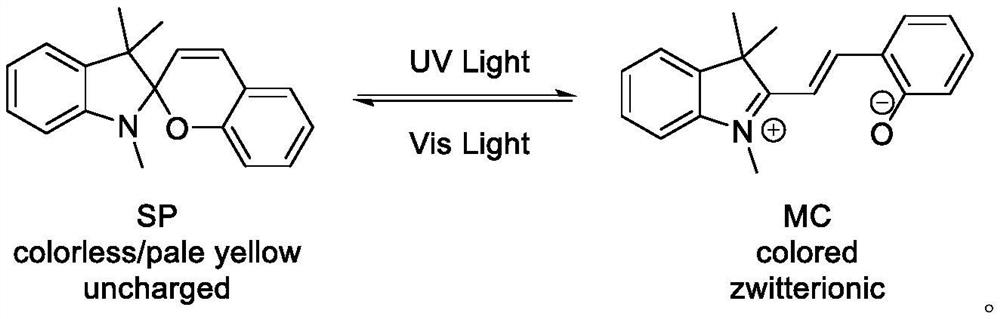
A technology of photochromic materials and spiropyrans, which is applied in the direction of color-changing fluorescent materials, chemical instruments and methods, organic chemistry, etc., can solve the problems of complex operation and unfriendly environment, and achieve simple reaction operation and high atom utilization rate , The effect of safe and low toxicity of raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
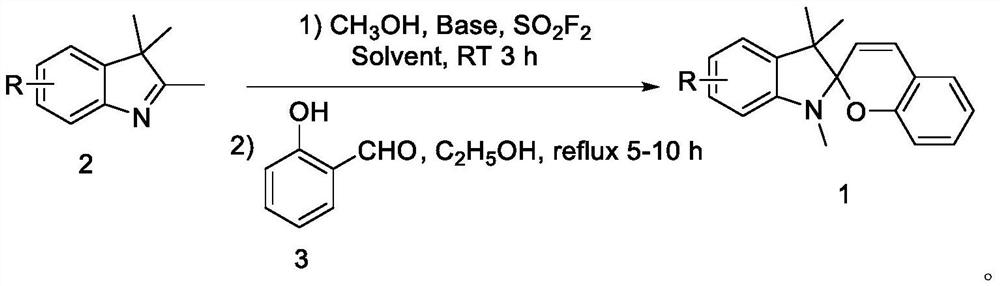
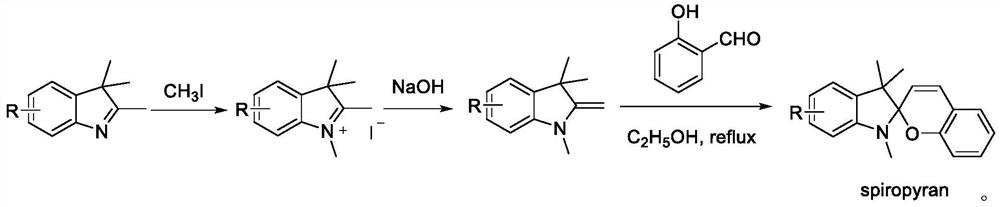
[0025] Example 1 Synthesis of 5'-fluoro-1',3',3'-trimethylspiro[chromene-2,2'-indoline]
[0026] In a 25mL dry three-necked flask, weigh sodium hydroxide (60mg, 1.5mmol, 3eq), 5-fluoro-2,3,3-trimethyl-3-H-indole (89mg, 0.5mmol), and use Sulfuryl fluoride replaced the gas in the reaction system; then added 1,4-dioxane (4mL), methanol (80μL, 2mmol, 4eq), and stirred at room temperature for 3 hours until the conversion of the raw materials was complete; then replaced the reaction system with nitrogen Gas, add ethanol (5mL) dehydrating agent, salicylaldehyde (52μL, 0.5mmol, 1eq), reflux and stir for 5 hours.
[0027] After the reaction was completed, it was quenched with water, extracted with dichloromethane, and the organic phase was washed with saturated brine, washed with Na 2 SO 4 Concentrated after drying, followed by silica gel column purification (PE:EA=50:1, R f =0.4) to obtain 105 mg of yellow solid, yield 71%.
[0028] 1 H NMR (500MHz, CDCl 3 )δ7.10(td, J=7.7,1.7Hz...
Embodiment 2
[0031] Example 2 Synthesis of 5'-fluoro-1',3',3'-trimethylspiro[chromene-2,2'-indoline]
[0032] The method is the same as in Example 1, except that sodium hydroxide is replaced by cesium carbonate, the solvent is dimethyl sulfoxide, and the yield is 65%.
Embodiment 3
[0033] Example 3 Synthesis of 5'-fluoro-1',3',3'-trimethylspiro[chromene-2,2'-indoline]
[0034] The method is the same as in Example 1, except that sodium hydroxide is replaced by potassium hydroxide, the solvent is dichloromethane, and the yield is 60%.
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap