Binding proteins to the human thrombin receptor, par4
A technology combining protein and thrombin, applied in the direction of anti-animal/human immunoglobulin, anti-receptor/cell surface antigen/cell surface determinant immunoglobulin, immunoglobulin, etc., can solve the unsatisfactory treatment of thrombosis. Medical needs, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0833] Example 1 Development of antagonist monoclonal antibody against human PAR4
[0834] The inventors tried to develop a monoclonal antibody targeting human PAR4. Antibody production and screening are performed at Monash Antibody Technology Facility (MATF) by its director Professor Mark Sleeman using Regeneron Pharmaceuticals' proprietary HumAb mice This was done to generate high-affinity antibodies against PAR4, as described in further detail below.
[0835] (i) Immunization
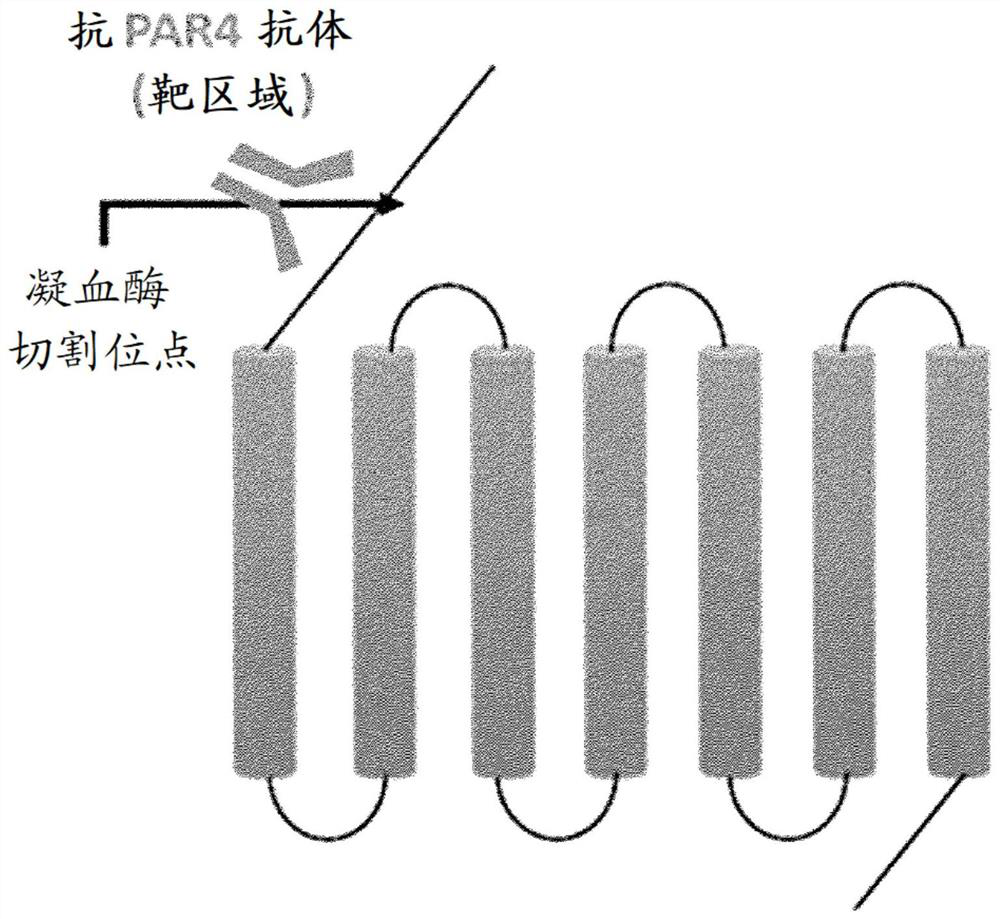
[0836] The KLH-conjugated peptide at the N-terminal thrombin cleavage and activation site of human PAR4 (hPAR4) was generated and used to immunize HumAb mice, followed by three booster immunizations according to the standard protocol. Serum titer was determined by ELISA using naked hPAR4 peptide (Table 3).
[0837] A total of 12 HumAb mice were subjected to three independent immunization programs using the peptide. The sequence of the peptide used for immunization is shown in Table 3, and in some cases ...
Embodiment 2
[0862] Example 2 Anti-PAR4 hybridoma clone blocks thrombin-induced PAR4 cleavage
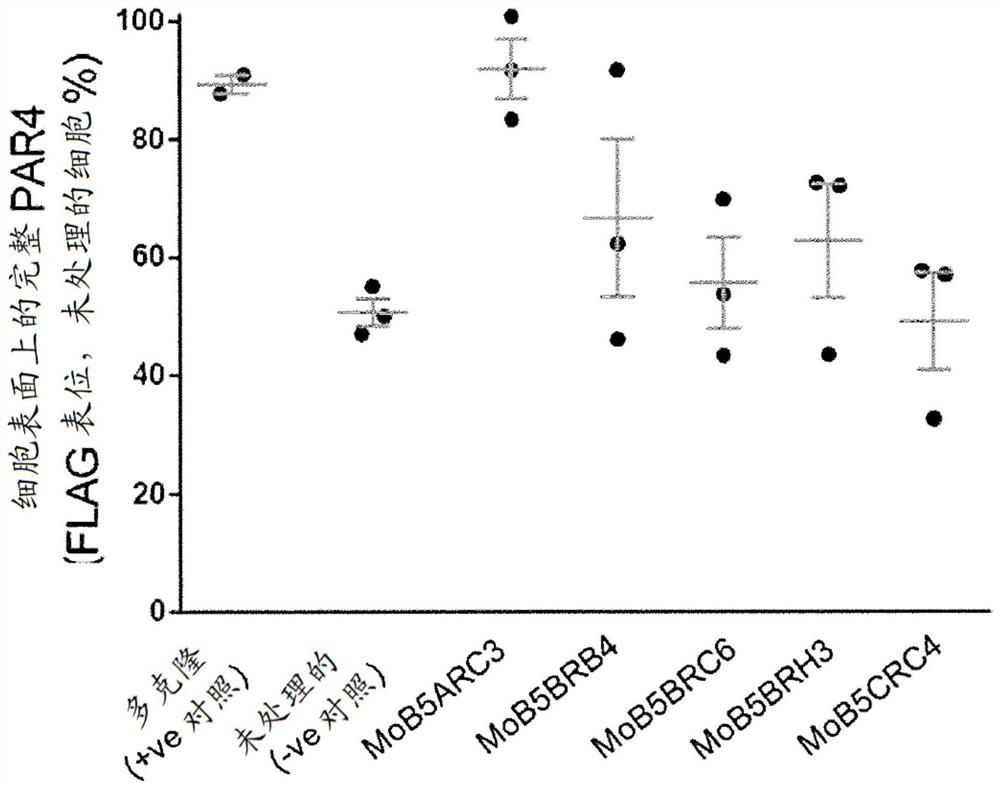
[0863] The ability of monoclonal antibody hybridoma supernatants (MoB5ARC3, MoB5BRB4, MoB5BRC6, MoB5BRH3 and MoB5CRC4) to cleave intact PAR4 present on the surface of HEK293 cells transfected with human PAR4 containing N-terminal FLAG tag was screened. By flow cytometry and as figure 2 Quantitative cutting shown.
[0864] Combine the transfected HEK293 cells with any anti-PAR4 polyclonal antibody (described in French SL et al. (2016) JThromb Haemost 14, 1642-1654, used as a positive control) or monoclonal antibody clones MoB5ARC3, MoB5BRB4, MoB5BRC6, MoB5BRH3 Or MoB5CRC4 and an untreated negative control are incubated for 10 minutes in the presence of thrombin (0.1U / ml) at room temperature. The cleavage of PAR4 by thrombin was measured by the loss of FLAG tags from PAR4-expressing HEK293T cells using flow cytometry.
[0865] Treatment of cells with thrombin (2U / ml for 10 minutes) resulted in approx...
Embodiment 3
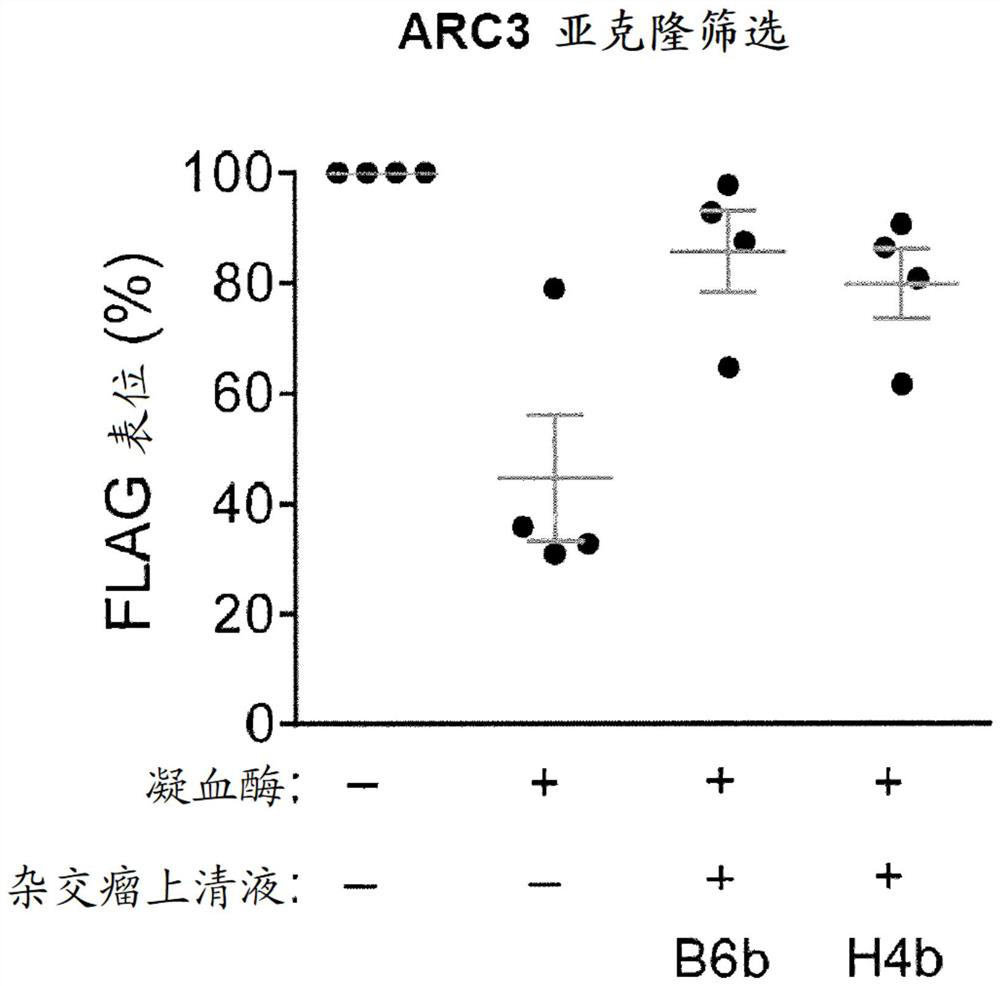
[0869] Example 3 Binding specificity of H4b and B6b subclones of mAb-5RC3
[0870] To determine the specificity of anti-hPAR4 clones for PAR4, ELISA screening was performed as described above. Figure 5 A shows the results of the binding specificity of clones mAb-5ARC3 (5A.RC3) and mAb-5BRB4 (5B.RB4) for hPAR1, hPAR2, hPAR3 and hPAR4.
[0871] 5A. RC3 shows 16-fold selectivity to human PAR4 peptide over PAR1, PAR2 and PAR3. Compared with 5A.RC3, mAb5B.RB4 similarly binds to all four human PAR peptides and has a lower affinity for PAR4.
[0872] The inventors used Bio-Rad Proteon XPR36 to perform surface plasmon resonance (SPR) analysis to gain insight into the binding affinity (association and dissociation rate) and specificity of 5A.RC3.F10b.H4b. Using the streptavidin chip, all biotin-conjugated human PAR peptides (Table 1) were captured on the surface, and different concentrations of purified 5A.RC3 passed. It was observed by SA chip SPR that clone 5A.RC3.F10b.H4b has a dissoci...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com