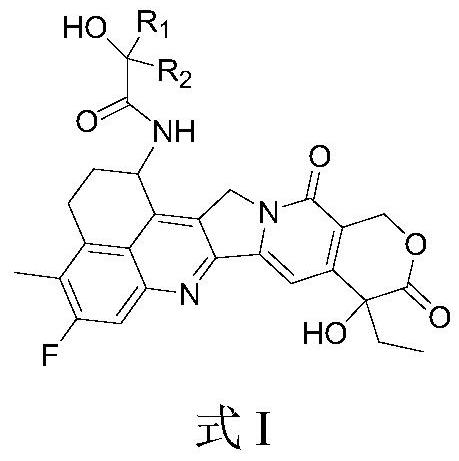
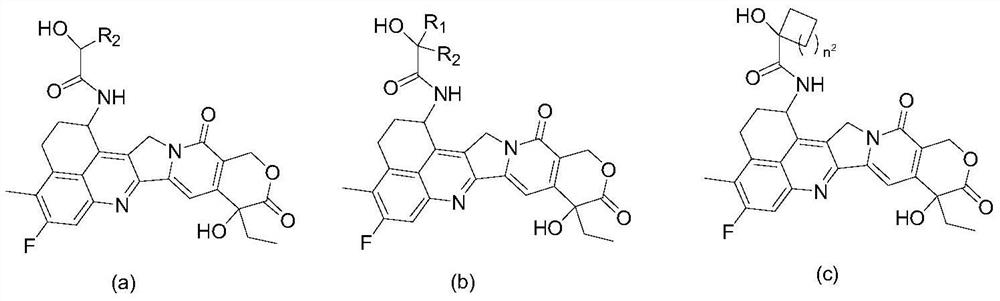
Camptothecin drugs and antibody conjugates thereof
A technology for camptothecin and antibody drugs, which can be used in drug combinations, anti-tumor drugs, pharmaceutical formulations, etc., and can solve problems such as narrow treatment windows
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0058] The synthesis of embodiment 1 compound 2
[0059]
[0060] Dissolve compound 1 (exitecan mesylate, purchased) (40mg, 75.3mmol, 1.0eq) and L-lactic acid (10mg, 113.0 mmol, 1.5eq) in dry 5mL DMF, then add PyBop (58.8mg , 113.0 mmol, 1.5 eq) and DIEA (15.7 uL, 113.0 mmol, 1.5 eq). After stirring at room temperature for 3 hours, TLC detected that the reaction was complete, quenched with water, extracted with dichloromethane (10 mL×3), combined the organic phases, dried over anhydrous sodium sulfate, filtered, the filtrate was concentrated under reduced pressure, and the residue was purified by column chromatography. Compound 2 (30.9 mg, 81.1%) was obtained. LC-MS: [M+H]+: 508.2. 1H NMR (400Mz, CDCl3 / CD3OD): 0.91-0.94 (3H, m), 1.32-1.39 (3H, m), 1.71-1.83 (2H, m), 2.31(3H,s),2.78-3.02(2H,m),3.16-3.26(2H,m), 4.27-4.35(1H,m),4.81-4.92(1H,m),5.15-5.24(2H,m ), 5.49-5.76 (2H, m), 7.52 (1H, d, J = 12.0Hz), 7.58 (1H, s), 7.75 (1H, d, J = 12.0Hz).
Embodiment 2
[0061] The synthesis of embodiment 2 compound 4
[0062]
[0063] Add compound 3 to a 500mL single-necked bottle: N-fluorenylmethoxycarbonyl-glycyl-glycine (10g, 28.2mmol, 1.0eq), lead tetraacetate (17.5g, 55.3mmol, 1.4eq), 200mL dry tetrahydrofuran and 67mL Toluene, stirred evenly, under nitrogen protection, heated to 85°C for 2.5h. TLC monitoring, after the raw material reacted, cooled to room temperature, filtered, the filtrate was concentrated under reduced pressure, and the residue was purified by column chromatography to obtain compound 4 (8.7 g, 83.7%)).
Embodiment 3
[0064] The synthesis of embodiment 3 compound 5
[0065]
[0066] Add compound 3 (500mg, 1.4mmol, 1.0eq), p-toluenesulfonic acid monohydrate (26mg, 0.1mmol, 0.1eq) and 10mL THF into a 25mL single-necked bottle, stir well, cool down to 0°C, and then slowly add L - Benzyl lactate (1.2g, 7.0mmol, 5eq), warmed up to room temperature after the addition was complete. TLC monitoring, after the reaction was completed, a saturated NaHCO3 solution was added, extracted with ethyl acetate, dried over anhydrous sodium sulfate, filtered, concentrated, and the residue was purified by a reverse-phase column to obtain compound 5 (400 mg, 60.3%). 1H NMR (400 Mz, CDCl3): 1.39 (3H, d, J = 6.8Hz), 3.78 (2H, t, J = 4.0Hz), 4.17-4.27 (2H, m), 4.42 (2H, d, J = 4.0Hz), 4.72-4.85(2H,m), 5.11-5.58(2H,m), 5.43(1H,s), 7.06(1H,t,J=8.0Hz), 7.25-7.33(6H,m), 7.38 (2H, t, J = 8.0Hz), 7.57 (2H, d, J = 8.0Hz), 7.75 (2H, d, J = 8.0Hz).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com