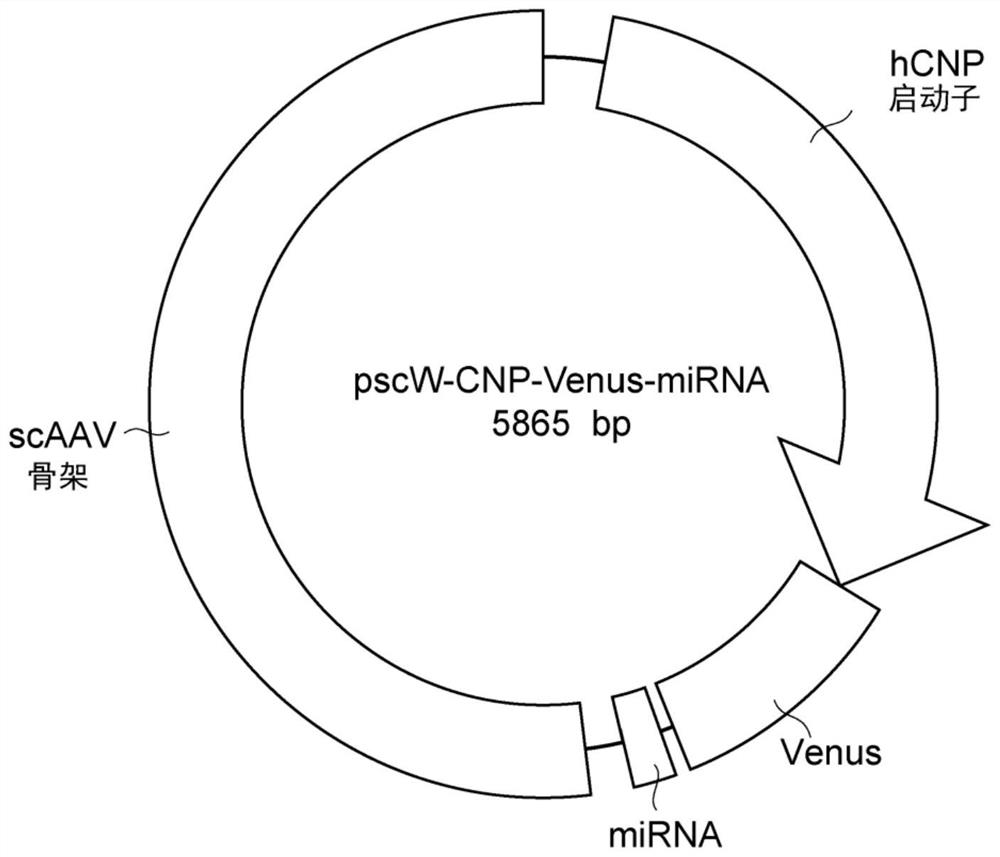
Oligodendrocyte-specific promoter, mirna specific to plp1 gene, vector including said promoter and/or mirna, and pharmaceutical composition including said vector
A technology of oligodendrocytes and PLP1, which is applied in the field of PLP1 gene-specific miRNA, can solve problems such as no records, and achieve the effect of inhibiting expression
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0134]
[0135] BLOCK-iT TM RNAi Designer (ThermoFisher Scientific, USA) was used to design a precursor miRNA sequence (PLP1 miRNA, sequence number 9) targeting the mRNA of the mouse PLP1 gene (NM_011123.3, sequence number 8) and as a negative control The precursor miRNA sequence (miR-neg, SEQ ID NO: 10). It should be noted that miR-neg is a miRNA sequence that is presumed to form a hairpin structure and be processed into a mature miRNA, but does not target a known vertebrate gene.
[0136] PLP1-miRNA (SEQ ID NO: 9):
[0137]5'-ACTCCAAAAGAAACACAATCCAGTTTTGGCCACTGACTGACTGGATTGTTTCTTTGGAGT-3'
[0138] miR-neg (sequence number 10):
[0139] 5'-GTATGCATCGAATGAGATTCCGTTTTGGCCACTGACTGACGGAATCTCTCGATGCATAC-3'
[0140] The PLP1 miRNA sequence and miR-neg sequence were synthesized according to the design, and cloned into the cloning site of the pcDNA (trademark) 6.2-GW / miR vector (ThermoFisherScientific, USA) contained in the BLOCK-iT (trademark) Pol IImiR RNAi expression vector k...
Embodiment 2A
[0148]
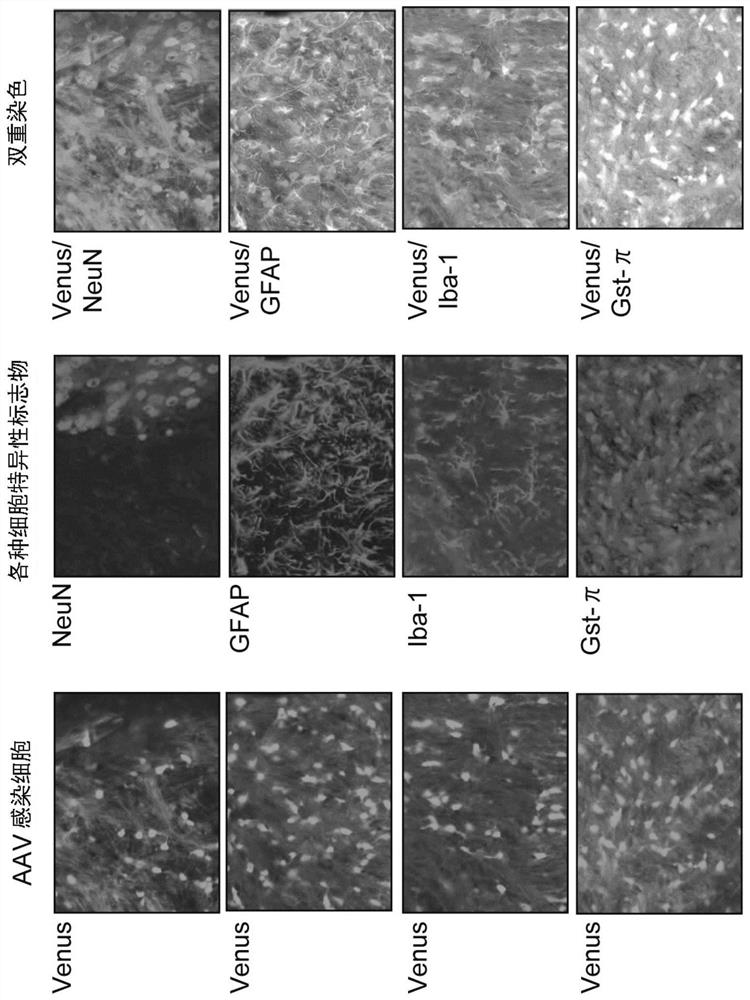
[0149] To the unilateral striatum and internal capsule of wild-type mouse Jcl on the 10th day after birth: B6C3F1, respectively inject 1 μl of the solution of the PLP1 miRNA carrier or miR-neg carrier obtained in Example 1 (titer: 1.2 ×10 12 vg / ml) (n=5 for each group). Before injection, mice were anesthetized with pentobarbital solution. Injection of the vector was performed at a rate of 150 nl / min using a 33G syringe. After injection, the needle was left in place for 1 minute, and the needle was slowly withdrawn from the brain. Afterwards, they were sent back to the mother mice in a group to raise them until the 17th day after birth.
[0150] Gene expression from the vector was assessed by detecting the green fluorescent protein Venus at postnatal day 17. Venus-expressing cells were identified by double immunostaining with cellular markers including Gst-π (mature oligodendrocytes), NeuN (neurons), Iba1 (microglia), and GFAP (astrocytes) kind of.
[0151] For...
Embodiment 3
[0153]
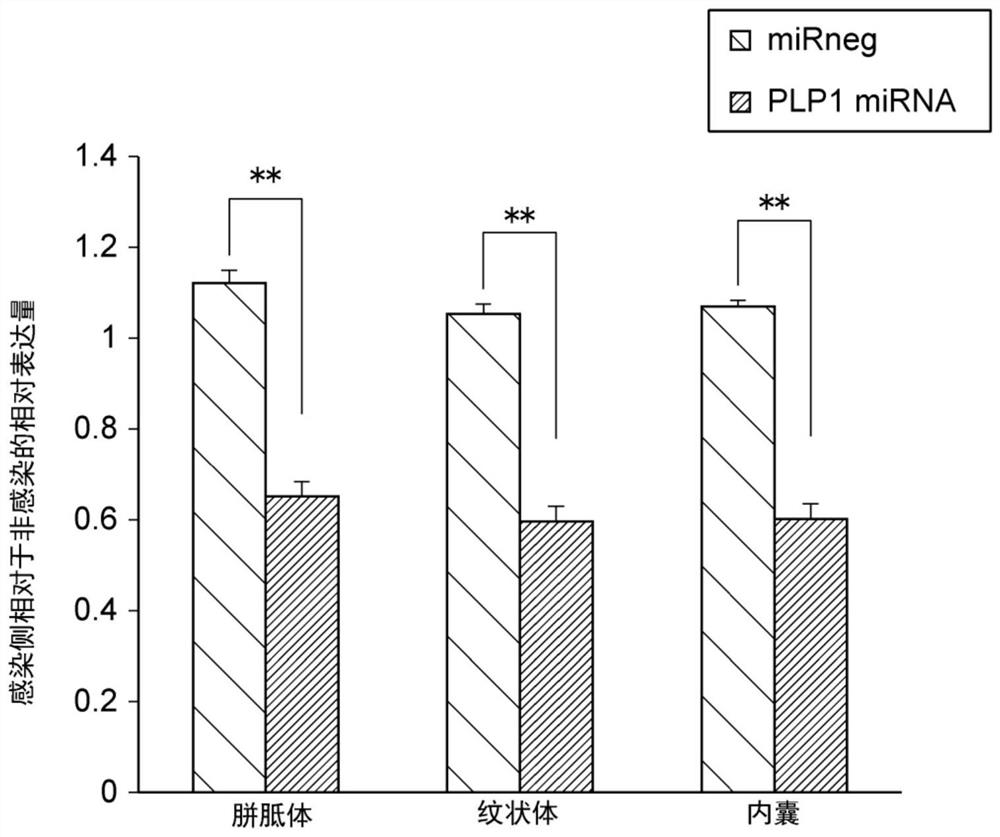
[0154] To the wild-type mouse Jcl on the 10th day after birth: B6C3F1 unilateral striatum, internal capsule and cerebellum respectively inject 1 μl of the solution of the two scAAV-AAV1 / 2 vectors obtained in Example 1 (titer : 1.2×10 12 vg / ml) (n=3 for each group). The injection method is the same as in Example 2. Thereafter, in order to evaluate the PLP1 miRNA knockdown efficiency, the expression of PLP1 protein in Venus-positive cells was investigated by immunostaining. Regarding the immunostaining method, an anti-PLP1 rabbit polyclonal antibody (Numata Y et al., J Biol Chem. 2013 Mar 15; 288(11): 7451-66) was used as the primary antibody, and an anti-rabbit fluorescent antibody (ThermoFisher Scientific, USA) was used. Except for the second antibody, it was carried out in the same manner as in Example 1. Nuclei of the corpus callosum, striatum, and internal capsule were photographed using a KEYENCE fluorescence microscope (KEYENCE, Japan).
[0155] The average...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com