Anti-cea antibody and application thereof, cell capable of secreting anti-cea antibody and preparation method thereof
An antibody and cell technology, applied in genetically modified cells, biochemical equipment and methods, cells modified by introducing foreign genetic material, etc., can solve the problem of poor specificity of anti-CEA antibodies
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0054] The method for preparing cells capable of secreting anti-CEA antibodies in one embodiment is characterized by comprising the following steps: using the mixture to transfect animal cells and culturing to obtain cells capable of secreting anti-CEA antibodies.
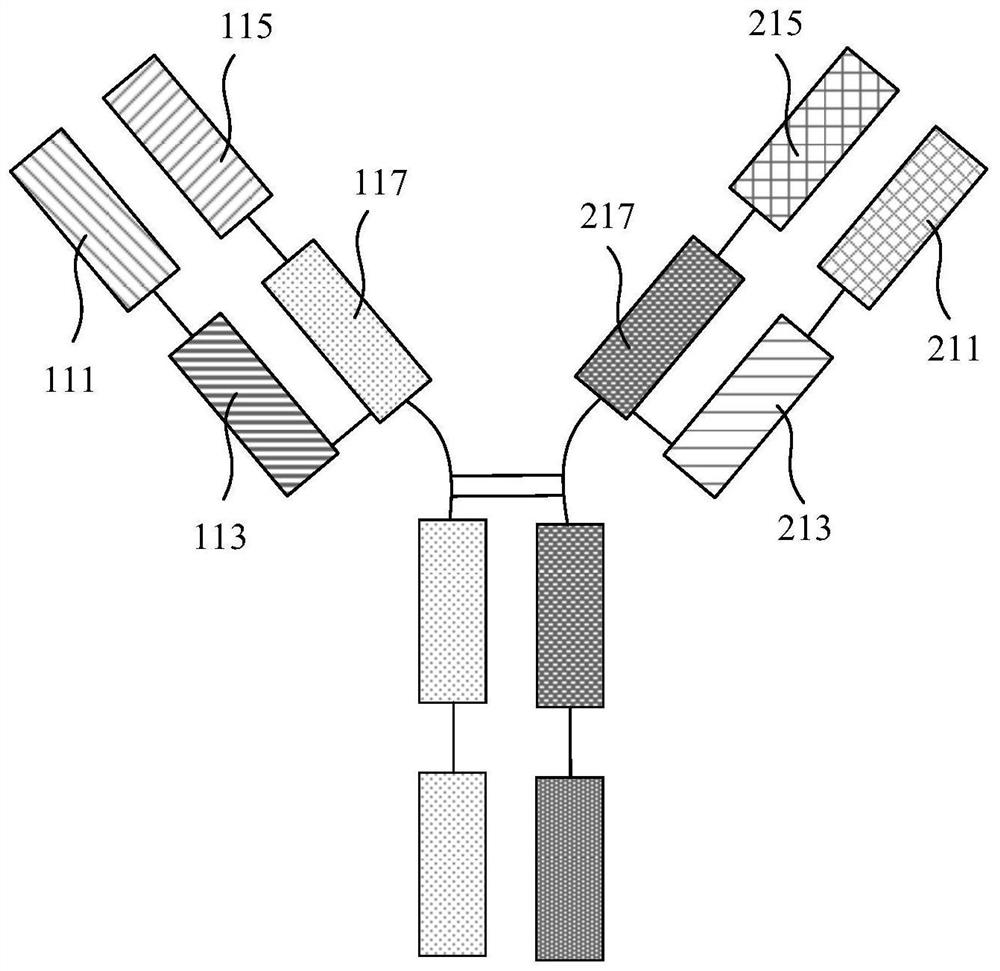
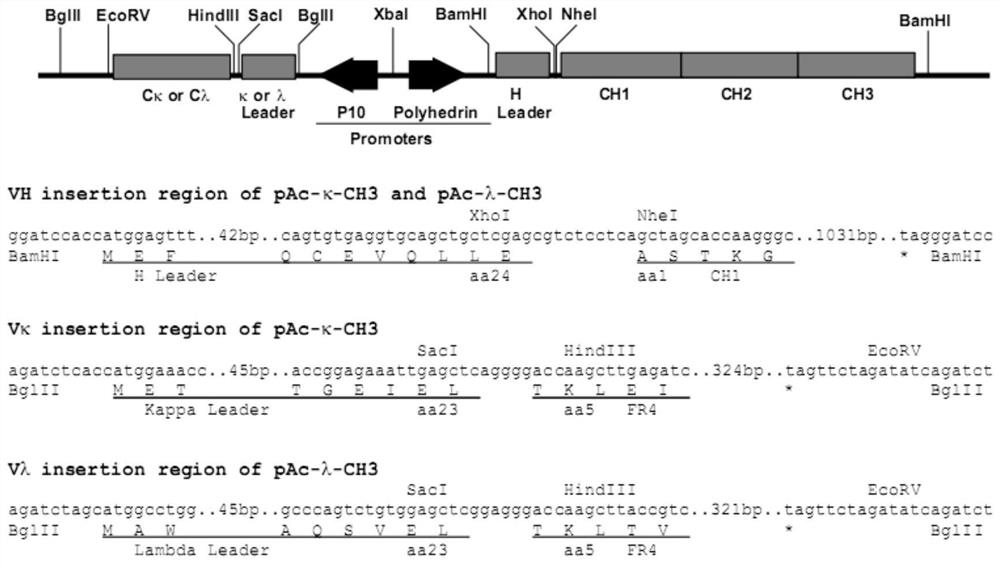
[0055] Wherein, the transfection composition comprises the first plasmid, the second plasmid and the transfection reagent. The first plasmid contains the coding sequence of the first light chain variable region and the coding sequence of the first heavy chain variable region, and the first light chain variable region The amino acid sequence of the region is shown in SEQ ID No.1, and the amino acid sequence of the first heavy chain variable region is shown in SEQ ID No.2. The second plasmid contains the coding sequence of the second light chain variable region and the coding sequence of the second heavy chain variable region, the amino acid sequence of the second light chain variable region is shown in SEQ ID No.3, a...
Embodiment 1
[0151] Monoclonal Antibody Preparation
[0152] Mix the immunogen and Freund's adjuvant in equal volumes and fully emulsify the mixture to obtain the mixture. Inject Balb / c mice with the mixture intraperitoneally to immunize Balb / c mice. A total of 3 immunizations, the interval between two adjacent immunizations For 15 days, the immunogen dose for each immunization was 25 μg / mouse.
[0153] After the immunization was completed, the spleen cells of the immunized mice were taken out, and 2.0×10 7 2.0×10 SP2 / 0 myeloma cells 8 Splenocytes of 1 immunized Balb / c mouse were mixed, centrifuged, the supernatant was discarded, slightly oscillated and mixed, placed in a water bath at 37°C, and 1 mL of 50% by volume PEG1500 aqueous solution was added dropwise within 90 seconds. Then add 20 mL of 1640 medium dropwise, centrifuge, discard the supernatant, wash once with PEG1500 aqueous solution and 1640 medium according to the above operation, centrifuge, discard the supernatant, and obta...
Embodiment 2
[0158] Antibody Titer Detection
[0159] The purified antibodies in Example 1 were diluted to concentrations of 3 μg / mL, 1 μg / mL, 0.33 μg / mL, 0.11 μg / mL, 0.036 μg / mL, and 0.012 μg / mL, and the antibody titer was detected by indirect ELISA method , ELISA test results are shown in Table 1. Wherein, the immunogen was diluted with CB buffer to a final concentration of 1 μg / mL to obtain a CB solution containing the immunogen. Add 100uL of CB solution containing immunogen to each well of the microtiter plate, and coat at 37°C for 3h. The microtiter plate was washed once with 0.01M PBST solution, and blocked by adding blocking solution (ie, PBS solution containing 20% fetal bovine serum by mass percentage) at 37° C. for 2 hours. Wash the microtiter plate once with 0.01M PBST solution. Add 100uL of diluted antibody to each well (that is, dilute the above-mentioned anti-CEA monoclonal antibody 1000 times, 3000 times, 9000 times, 27000 times, 81000 times with ascites) and react at 3...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com