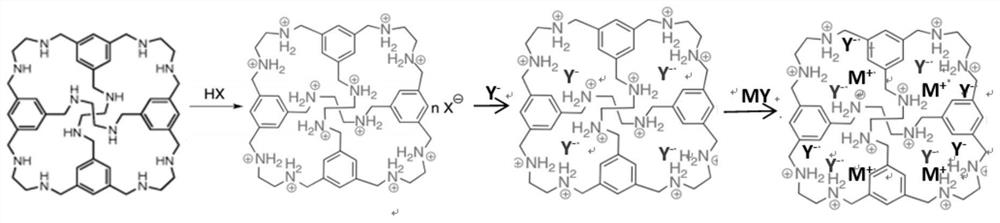
A kind of porous organic compound electrolyte and its preparation method and application
An organic compound and electrolyte technology, applied in the field of electrochemistry, can solve the problems of low room temperature ionic conductivity, high cost, harsh preparation conditions, etc., and achieve the effect of realizing ion conduction, increasing the degree of freedom, and high ion migration number
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
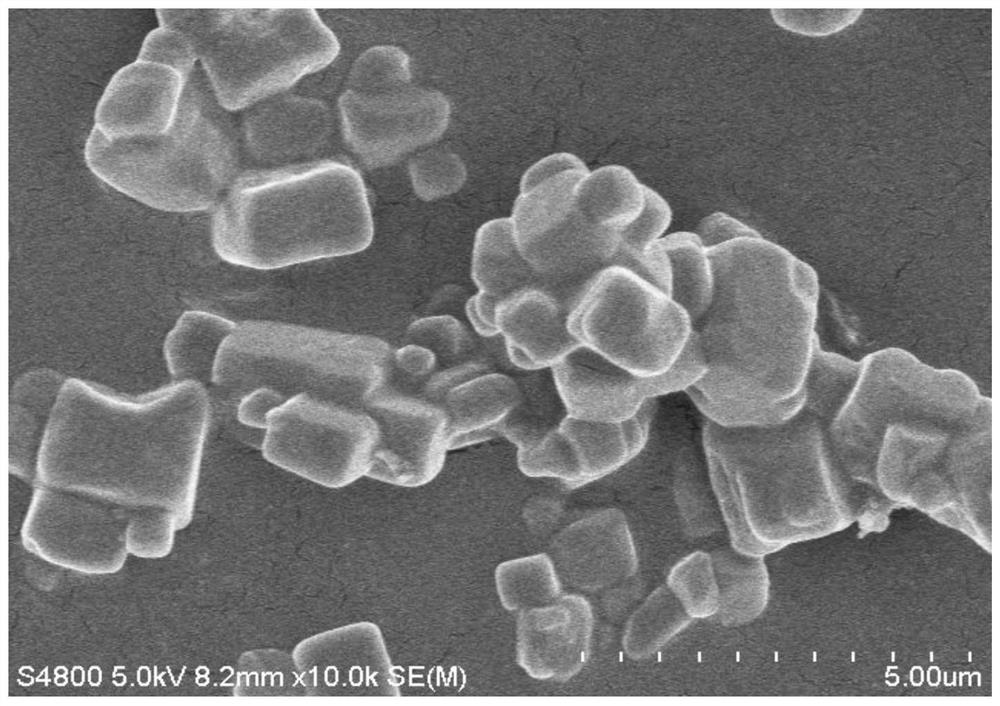
[0046] Dissolve 1 g of the laboratory-prepared polyamine macrocyclic porous organic compound RCC1 powder in 10 mL of chloroform. Add 3 mL of commercially available 4 M hydrochloric acid / dioxane solution dropwise to the above solution to obtain white precipitate RCC1-Cl, wash the precipitate and add 10 mL of 10% mass fraction lithium perchlorate-ethanol solution, heat and stir . Replace the lithium perchlorate-ethanol solution twice in the middle to obtain RCC1-ClO 4 . 1g RCC1-ClO 4 Composite with 0.68g lithium perchlorate to obtain porous organic compound electrolyte Li-RCC1-ClO 4 . Its electron microscope appearance is as image 3 As shown, the macroscopic photos after tableting are as follows Figure 4 As shown, the conductivity as Figure 5 As shown, the electrochemical window as Figure 6 As shown, thermal stability as Figure 7 shown.
Embodiment 2
[0048] Dissolve 1 g of the laboratory-prepared polyamine macrocyclic porous organic compound TpEDA powder in 10 mL of N,N-dimethylformamide (DMF). Add 5 mL of commercially available 2 M sulfuric acid / dioxane solution dropwise to the above solution to obtain brown-red precipitate TpEDA-SO 4 After washing the precipitate, add 10 mL of 10% by mass lithium hexafluorophosphate-ethyl acetate solution, heat and stir. Replace the lithium hexafluorophosphate-ethyl acetate solution twice in the middle to obtain TpEDA-PF 6 . 2g TpEDA-PF 6 Composite with 1.3g lithium hexafluorophosphate to obtain porous organic compound electrolyte Li-TpEDA-PF 6 .
Embodiment 3
[0050] Dissolve 1 g of the laboratory-prepared polyamine macrocyclic porous organic compound TpPNDA powder in 10 mL of dimethyl sulfoxide (DMSO). Add 3 mL of commercially available 4 M sulfuric acid / dioxane solution dropwise to the above solution to obtain a brownish-yellow precipitate TpPNDA-Cl, wash the precipitate and add 10 mL of 10% mass fraction lithium trifluoromethanesulfonimide - n-butanol solution, heated and stirred. The lithium trifluoromethanesulfonimide-n-butanol solution was replaced twice in the middle to obtain TpPNDA-TFSI. 5g TpPNDA-TFSI was combined with 3.8g lithium trifluoromethanesulfonimide to obtain a porous organic compound electrolyte Li-TpPNDA-TFSI.
PUM
| Property | Measurement | Unit |
|---|---|---|
| electrical conductivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com