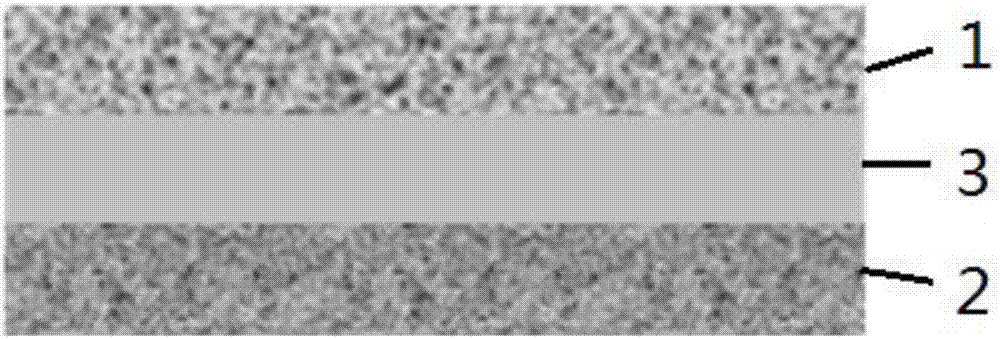
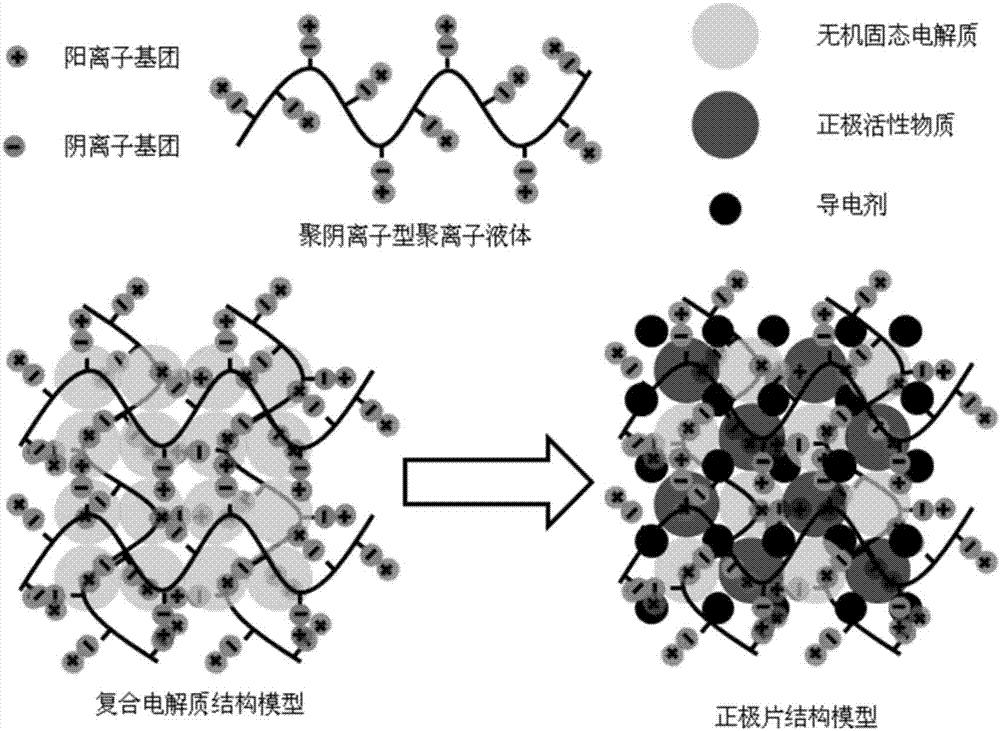
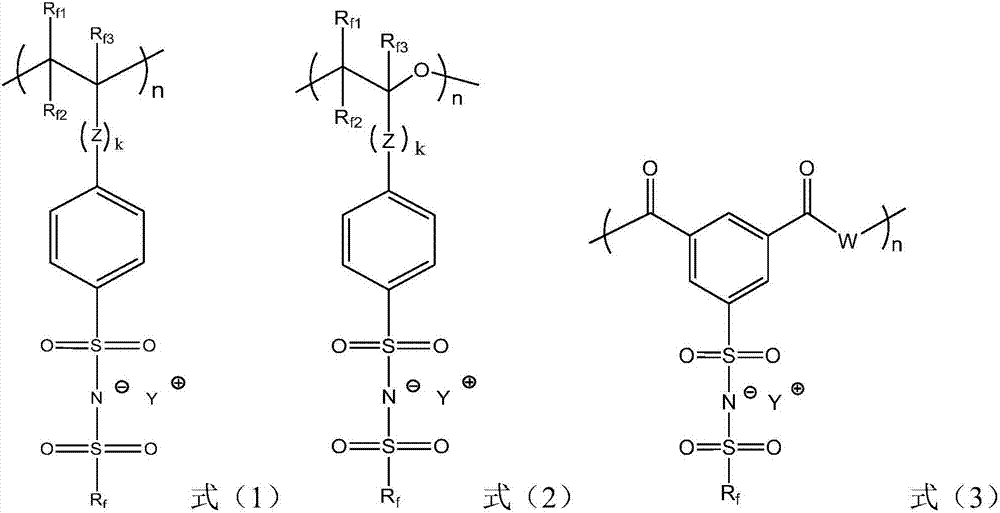
Composite solid electrolyte and solid-stage battery
A solid electrolyte and polymer technology, applied in non-aqueous electrolyte batteries, secondary batteries, circuits, etc., can solve the problems of slow ion migration and low conductivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0051] The preparation method of the composite solid electrolyte can be a preparation method well known to those skilled in the art, for example, the composite solid electrolyte can be prepared according to the following steps:
[0052] (1) dissolving and mixing the polymer ionic liquid and the inorganic solid electrolyte in a solvent to obtain an electrolyte solution;
[0053] (2) Disperse the electrolyte solution evenly on the Teflon plate and volatilize the solvent to obtain a composite solid electrolyte.
[0054] Wherein, the solvent may be at least one selected from acetonitrile, dimethyl sulfoxide, tetrahydrofuran and N,N-dimethylformamide.
[0055] Wherein, in order to reduce the impact of environmental impurities and further improve the conductivity of the composite solid electrolyte, the environmental conditions in the preparation process are preferably: H 2 O content is less than 0.5ppm, O 2 The content is less than 0.5ppm.
[0056] The present disclosure also pro...
Embodiment 1
[0077] (1) Preparation of ionic liquid polymer:
[0078]
[0079] Take 2.0266g (10mmol) of p-vinylbenzenesulfonamide, 2.3794g (20mmol) of thionyl chloride, and 1.3982g (12mmol) of chlorosulfonic acid at 100°C for 12h to obtain compound 1a (2.5357g, yield 90%) ; 1 H NMR (400MHz, CDCl 3 , ppm), δ=7.88(d,2×1H), 7.58(d,2×1H), 6.63(q,1H), 5.61(q,1H), 5.18(q,1H), 2.0(s,1H );
[0080] Take 2.8174g (10mmol) of compound 1a and 2.1451g (12mmol) of SbF 3 Reacted at 60°C for 12h to obtain compound 1b (2.3875g, yield 90%); 1 H NMR (400MHz, CDCl 3 , ppm), δ=7.88(d,2×1H), 7.58(d,2×1H), 6.63(q,1H), 5.61(q,1H), 5.18(q,1H), 2.0(s,1H );
[0081] Take 2.6528g (10mmol) of compound 1b and 1.3821g (10mmol) K 2 CO 3 Reacted at 25°C for 2h to obtain compound 1c (3.0337g, yield 100%); 1 H NMR (400MHz, CDCl 3 , ppm), δ=7.88(d,2×1H), 7.58(d,2×1H), 6.63(q,1H), 5.61(q,1H), 5.18(q,1H);
[0082] Take 3.0337g (10mmol) of compound 1c and 1.6128g (11mmol) of 1-ethyl-3-methylimidazole chloride to ...
Embodiment 2
[0091] (1) Preparation of ionic liquid polymer:
[0092]
[0093] Take 2.0266g (10mmol) of p-vinylbenzenesulfonamide, 2.3794g (20mmol) of thionyl chloride, and 1.3982g (12mmol) of chlorosulfonic acid at 100°C for 12h to obtain compound 2a (2.5357g, yield 90%) ; 1 H NMR (400MHz, CDCl 3 , ppm), δ=7.88(d,2×1H), 7.58(d,2×1H), 6.63(q,1H), 5.61(q,1H), 5.18(q,1H), 2.0(s,1H );
[0094] Take 2.8174g (10mmol) of compound 2a and 2.1451g (12mmol) of SbF 3 Reacted at 60°C for 12h to obtain compound 2b (2.3875g, yield 90%); 1 H NMR (400MHz, CDCl 3 , ppm), δ=7.88(d,2×1H), 7.58(d,2×1H), 6.63(q,1H), 5.61(q,1H), 5.18(q,1H), 2.0(s,1H );
[0095] Take 2.6528g (10mmol) of compound 2b and 1.3812g (10mmol) PhCO 3 H, 1.3821g (10mmol)K 2 CO 3 Reacted at 25°C for 12h to obtain compound 2c (2.8743g, yield 90%); 1 H NMR (400MHz, CDCl 3 , ppm), δ=7.86(d,2×1H), 7.47(d,2×1H), 3.82(t,1H), 2.83(d,2H);
[0096] Take 3.1937g (10mmol) of compound 2c and 1.6128g (11mmol) of 1-ethyl-3-methylimidazo...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com