Oligonucleotide and application thereof
An oligonucleotide and polynucleotide technology, which is applied in the application, medical preparations with non-active ingredients, and medical preparations containing active ingredients, etc., to achieve the effects of relieving corneal dystrophy lesions, preventing deterioration and preventing the occurrence of diseases
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0105] Example 1: Luciferase reporter system screening for efficient RNAi target sequences
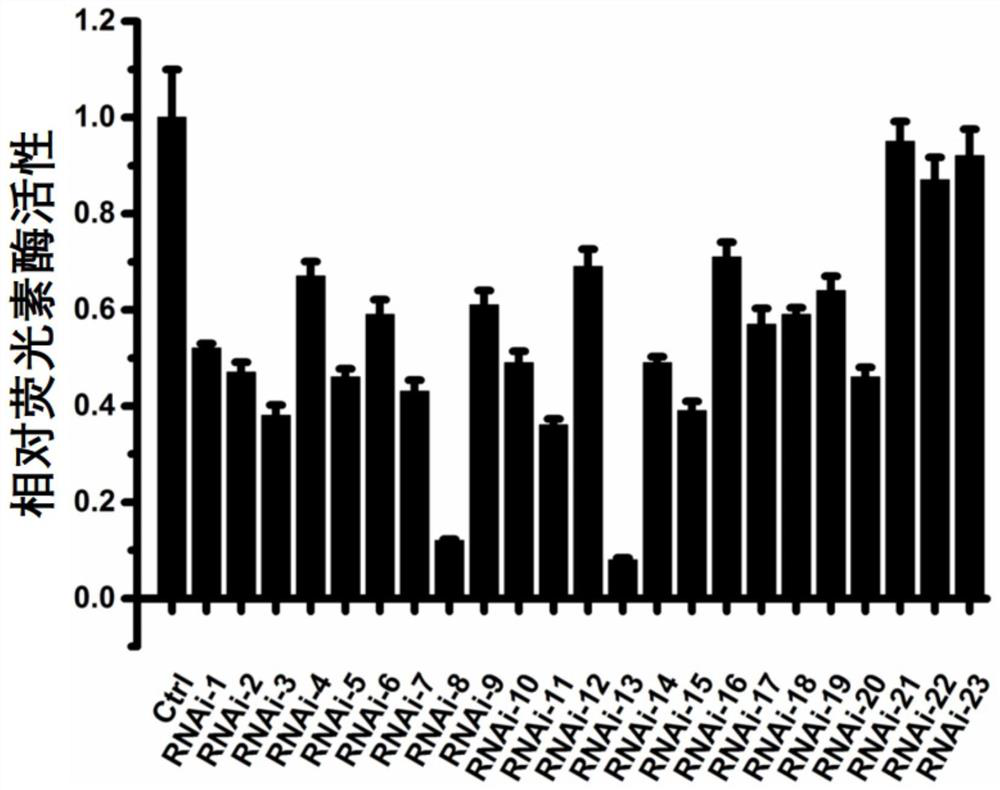
[0106] In 293 cells, luciferase plasmids containing mutant TCF4 sequences were co-transfected with RNAi controls (random sequences not targeting TCF4: CCGCCTTTTGAAGGCAAGTC, random sequences used in any of the following controls) or candidate RNAi drugs (including 20 oligonucleotides of the present invention) 23 oligonucleotides targeting intron 2 of TCF4 including nucleotides, please refer to the plasmid map figure 1 A and figure 1 B). After 48 hours of transfection, the luciferase activity was detected, and it was found that compared with the transfection RNAi control, the 20 oligonucleotides provided by the present invention had a significant inhibitory effect on the luciferase activity of mutant TCF4 ( figure 2 middle RNAi-1-20), while the remaining 3 oligonucleotides had no significant inhibitory effect on TCF4 RNA levels. This indicates that the oligonucleotides provided by th...
Embodiment 2
[0107] Example 2: RNAi drug treatment reduces intron 2 RNA foci of mutated TCF4
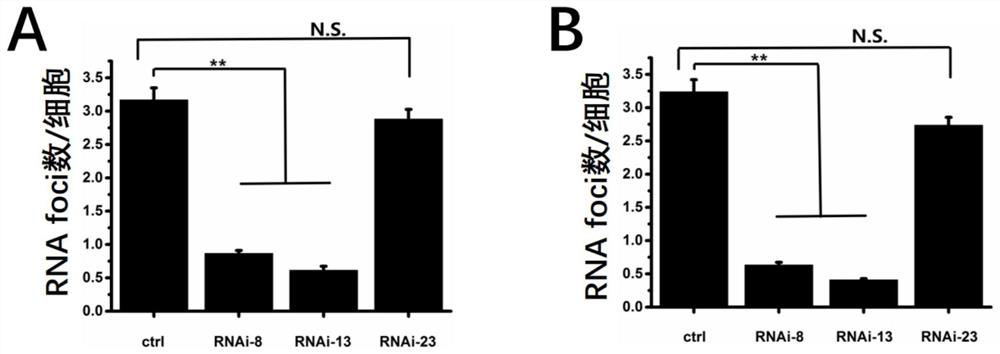
[0108] AAV infects 293 cells: the recombinant virus AAV2-ctrl (carrying a random sequence that does not target TCF4 is a virus vector of AAV2), AAV2-RNAi-X (carrying an oligonucleotide serotype is a virus vector of AAV2, X represents the serial number of the aforementioned 1-23 oligonucleotides, the same below) The medicine is MOI=1*10 4 The multiplicity of infection of vg / cell infected 293 cells. After 48 hours of infection, the cells were sampled to detect the RNA foci content.
[0109] Using the CRISPR-Cas9 method, a stable cell line with CTG repeat expansion in intron 2 of 293-TCF4 was constructed. In the TCF4 mutant cells, the chemically synthesized RNAi-X drug (i.e. the aforementioned oligonucleotide, X represents the sequence number of the aforementioned 1-23 oligonucleotides, the same below) and the ctrl control (the aforementioned random sequence) were transfected. After 24 hours of t...
Embodiment 3
[0111] Example 3: RNAi drugs have no significant effect on the expression level of wild-type TCF4 gene
[0112] AAV infection of B4G12 cells: the recombinant virus AAV2-ctrl, AAV2-RNAi-X drug at MOI=1*10 4 B4G12 cells were infected with a multiplicity of infection of vg / cell. 48h after infection, RNA was extracted to detect the content of TCF4 protein.
[0113] Although the oligonucleotides of the present invention target the No. 2 intron RNA of the TCF4 gene as RNAi drugs, they will not affect the protein expression level of TCF4 in theory. In order to confirm whether the drug treatment has an impact on the expression level of the TCF4 protein, this Examples are verified. B4G12 cells were transiently transfected with random sequence control or RNAi-13 drug, and the protein level of TCF4 was detected by Western Blot 24 hours later. Compared with random sequence control, RNAi-13 drug treatment did not significantly change the protein expression of TCF4 ( Figure 4 A).
[0...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap