Dry immunofluorescence quantitative method heparin binding protein (HBP) detection kit
A heparin-binding protein and detection kit technology, applied in the field of in vitro diagnostic immunological assay analysis, can solve the problems of low accuracy and stability, inability to perform bedside detection, harsh storage conditions, etc. Difficulty, low cost effect of inconsistent storage conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment 1
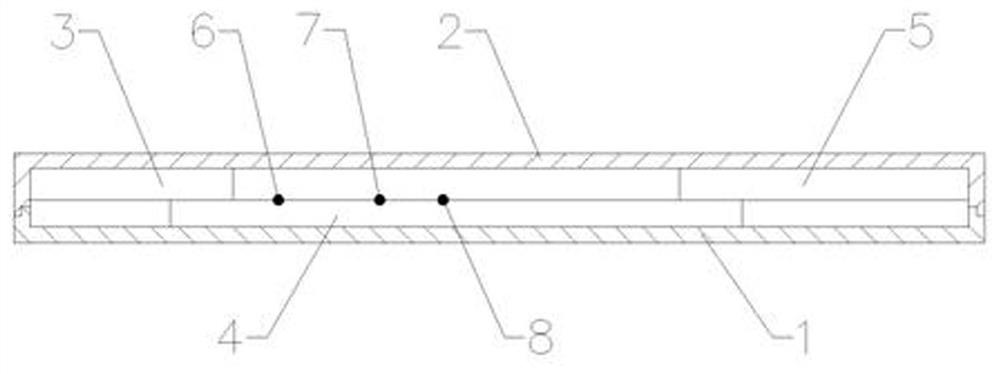
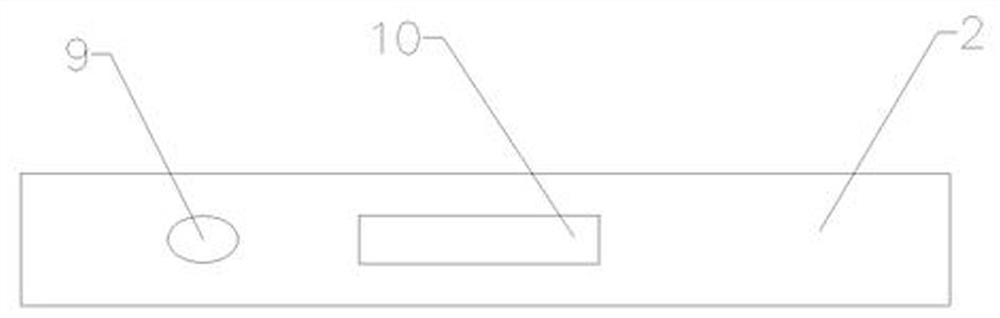
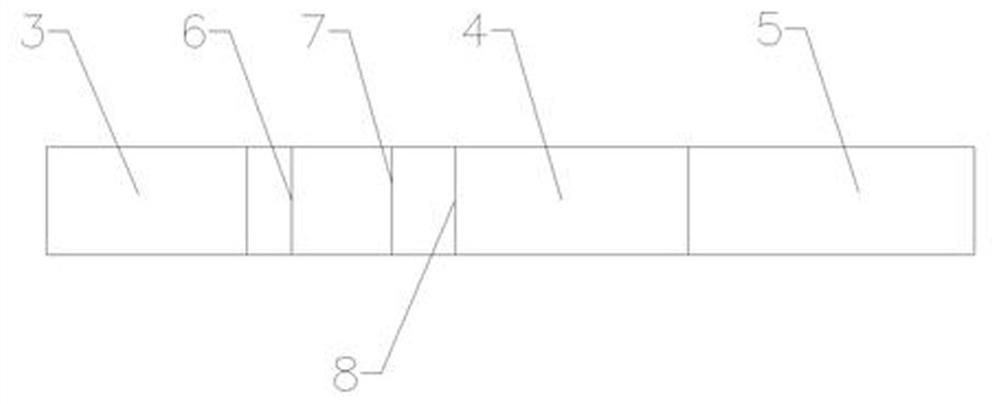
[0035] Such as figure 1 , figure 2 and image 3 As shown, a dry-type immunofluorescence quantitative method heparin-binding protein (HBP) detection kit, including a lower cover and an upper cover 2 and a reagent strip, the lower cover 1 and the upper cover 2 are snap-connected to each other, and the lower cover 1 and the upper cover 2 An accommodating cavity is formed between them, and the reagent strip is located in the accommodating cavity, and the upper cover 2 is provided with a sampling hole 9 and a detection window 10, and the reagent strip includes an absorbent paper 5, a nitrocellulose membrane 4 and a sample pad 3, and the absorbent The paper 5 is located at one end of the nitrocellulose membrane 4, the sample pad 3 is located at the other end of the nitrocellulose membrane 4, and the nitrocellulose membrane 4 is sequentially coated with a quality control C line 8, a detection T line 7, and a fluorescent The antibody solid phase line 6, the sample injection hole 9 ...
Embodiment 2
[0053] Such as figure 1 , figure 2 and image 3 As shown, a dry-type immunofluorescence quantitative method heparin-binding protein (HBP) detection kit, including a lower cover and an upper cover 2 and a reagent strip, the lower cover 1 and the upper cover 2 are snap-connected to each other, and the lower cover 1 and the upper cover 2 An accommodating cavity is formed between them, and the reagent strip is located in the accommodating cavity, and the upper cover 2 is provided with a sampling hole 9 and a detection window 10, and the reagent strip includes an absorbent paper 5, a nitrocellulose membrane 4 and a sample pad 3, and the absorbent The paper 5 is located at one end of the nitrocellulose membrane 4, the sample pad 3 is located at the other end of the nitrocellulose membrane 4, and the nitrocellulose membrane 4 is sequentially coated with a quality control C line 8, a detection T line 7, and a fluorescent The antibody solid phase line 6, the sample injection hole 9 ...
Embodiment 3
[0070] Embodiment 3: as figure 1 , figure 2 and image 3 As shown, a dry-type immunofluorescence quantitative method heparin-binding protein (HBP) detection kit, including a lower cover and an upper cover 2 and a reagent strip, the lower cover 1 and the upper cover 2 are snap-connected to each other, and the lower cover 1 and the upper cover 2 An accommodating cavity is formed between them, and the reagent strip is located in the accommodating cavity, and the upper cover 2 is provided with a sampling hole 9 and a detection window 10, and the reagent strip includes an absorbent paper 5, a nitrocellulose membrane 4 and a sample pad 3, and the absorbent The paper 5 is located at one end of the nitrocellulose membrane 4, the sample pad 3 is located at the other end of the nitrocellulose membrane 4, and the nitrocellulose membrane 4 is sequentially coated with a quality control C line 8, a detection T line 7, and a fluorescent The antibody solid phase line 6, the sample injectio...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com