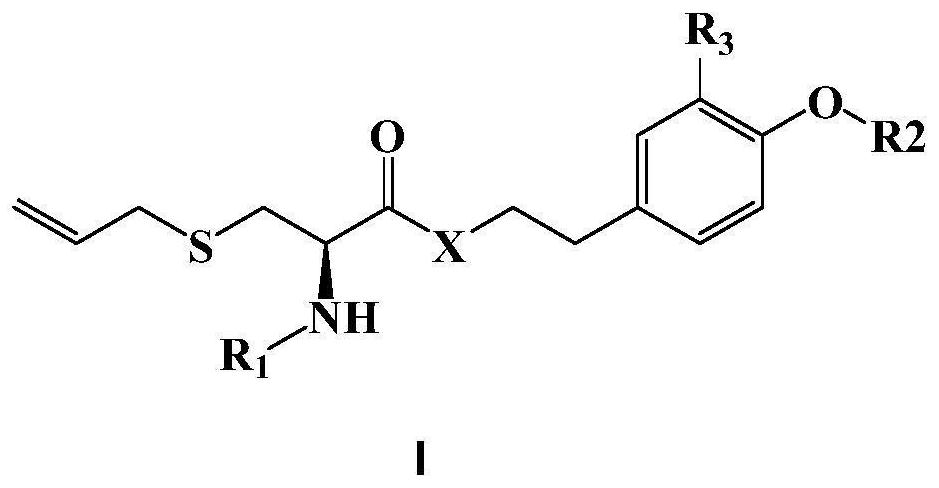
Synthesis method and application of S-allyl-L-cysteine substituted tyrosol derivative with neuroprotective activity
A technology of cysteine and derivatives, applied in the field of medicinal chemistry, can solve the problems of limiting tyrosol application, low bioavailability, unstable absorption, etc., and achieve the goal of improving mitochondrial function, improving glucose metabolism, and inhibiting cell apoptosis Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Preparation process of compounds SAO-1~SAO-8, SAO-9~SAO-16.
[0038] Synthesis of compound 1-1
[0039]
[0040] Compound 1-0 (16.1g, 100mmol) was weighed and dissolved in tetrahydrofuran (100ml), di-tert-butyl dicarbonate (21.8g, 100mmol) and saturated sodium bicarbonate solution (250ml) were added successively, and stirred at room temperature for 5 hours. After the reaction, the pH was adjusted to acidic with dilute hydrochloric acid (1 mol / L), then extracted with ethyl acetate, the organic phase was dried over anhydrous sodium sulfate, filtered, and concentrated to obtain product 1-1 (22.2 g, 85%). MS(ESI)m / z:260.1[M-H] - .
[0041] Synthesis of Compound 1-2
[0042]
[0043] Under nitrogen protection, compound 1-1 (2.61g, 10mmol), triphenylphosphine (2.62g, 10mmol), tyrosol (1.38g, 10mmol) were dissolved in anhydrous tetrahydrofuran (40ml), and added dropwise under ice-bath conditions Diisopropyl azodicarboxylate (2.02g, 10mmol) was reacted at room tempera...
Embodiment 2
[0114] Activity evaluation experiment of the compound of the present invention
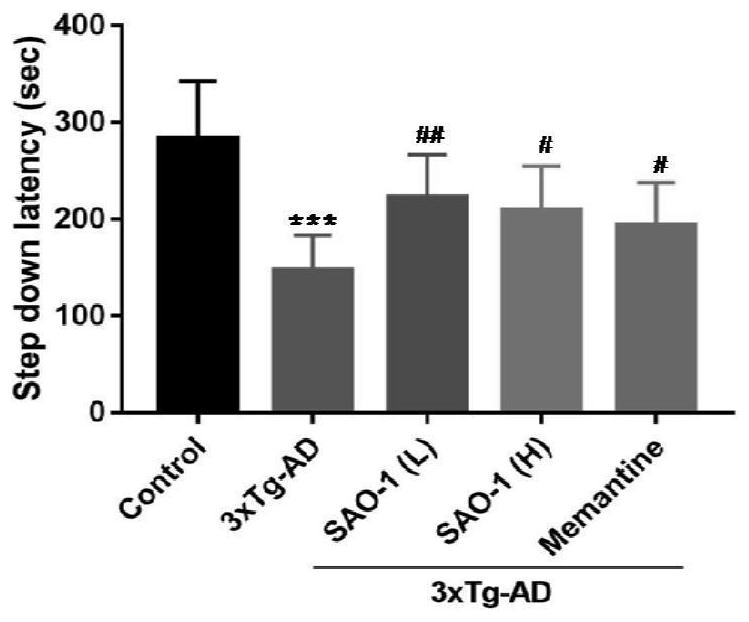
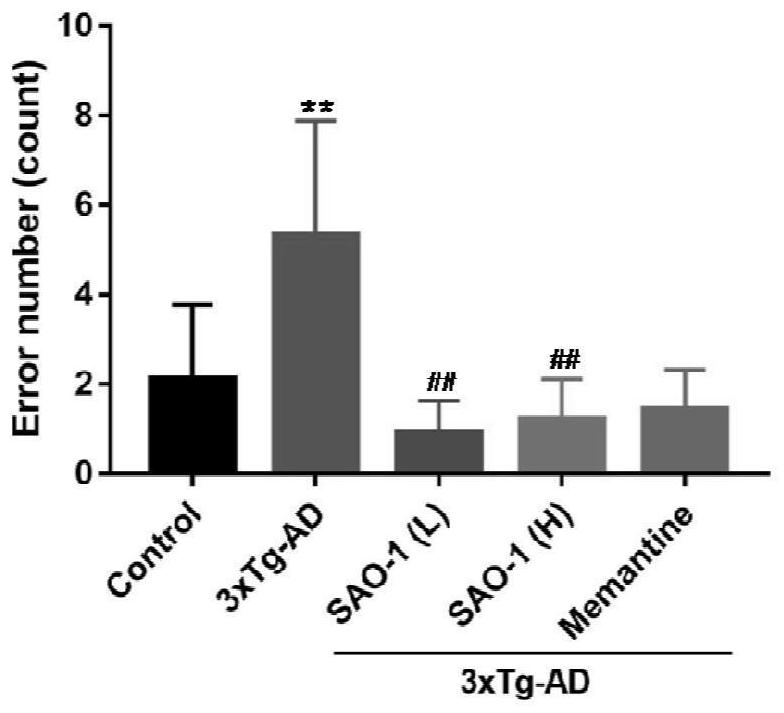
[0115] After Alzheimer's disease transgenic mice (3xTg-AD, 8 months old) were treated with compound SAO-1 (5.0mg / kg and 20.0mg / kg) for 4 weeks, behavioral tests found that the synthetic representative small molecule compound SAO-1 can significantly improve the platform latency of mice (A) and reduce the number of platform mistakes (B), and the effect is more obvious than the positive control drug memantine (5.0mg / kg), suggesting that SAO-1 can significantly Improved spatial memory in Alzheimer's disease transgenic mice.
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap