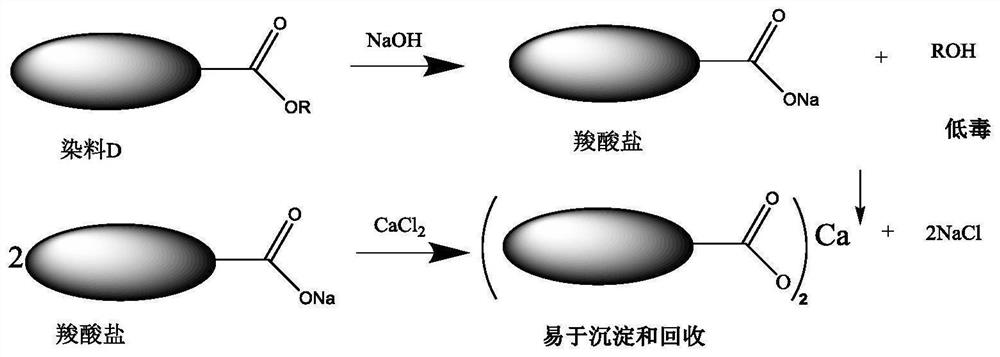
Recovery method of alkali-washable disperse dye hydrolysate
A technology of disperse dyes and hydrolyzate, applied in the direction of organic dyes, water pollutants, azo dyes, etc., can solve the problem of large acid consumption, and achieve the effect of cost saving
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-1
[0055] Embodiment 1-1, a method for recovering the hydrolyzate of alkali-washable disperse dyes, for the cloth sample (fabric) dyed with red 1 dye, carry out alkaline washing solution obtained by alkali washing in 0.1g / L NaOH aqueous solution; after testing Calculate, in this alkaline washing solution, the molar concentration of the carboxylate dye as hydrolysis product is 2.9mmol / L.
[0056] The carboxylate dye formed after the red 1 dye is washed with alkali is
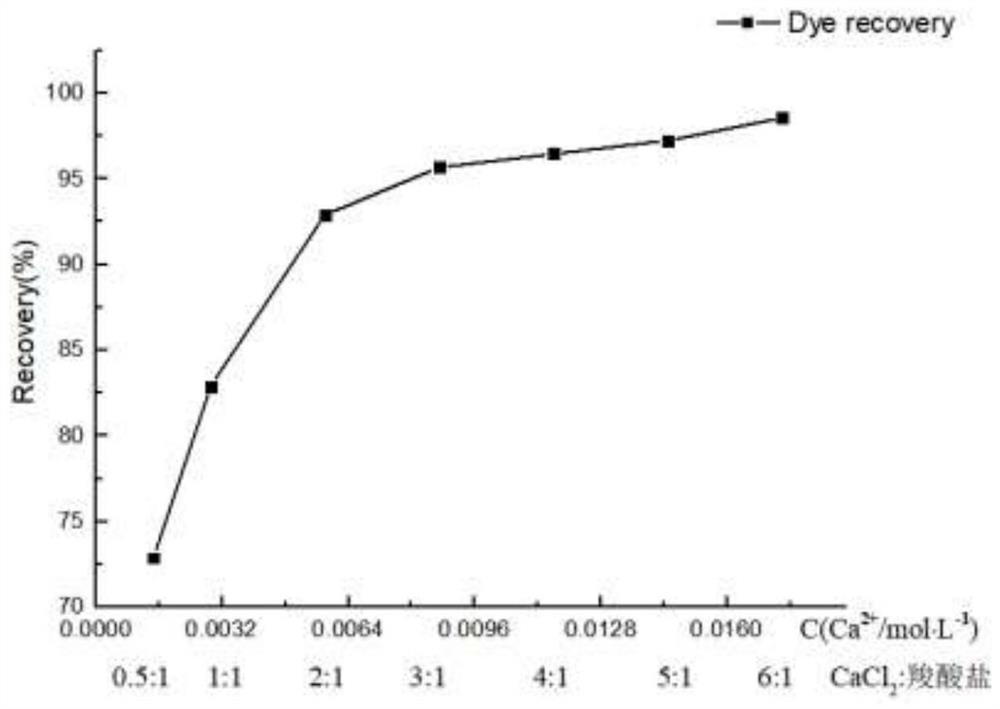
[0057] Add different amounts of calcium chloride saturated solution to the alkali washing solution, so that the molar ratio of calcium chloride: carboxylate dye is 0.5:1, 1:1, 2:1, 3:1, 4:1, 5:1, 6:1, after fully stirring for 1 hour, let stand for 24 hours. The recovery curve of carboxylate dye precipitation in different molar ratios, such as figure 2 shown.
[0058] When the molar ratio of calcium chloride to carboxylate dye reaches 2:1, that is, when the calcium ion concentration is 5.8mmol / L, the recovery r...
Embodiment 1-2
[0060] Embodiment 1-2, the concentration of NaOH in the NaOH aqueous solution is changed into 1g / L; The molar concentration of carboxylate dyestuff is controlled as 2.9mmol / L in the alkali washing liquid; All the other are equal to embodiment 1-1; When calcium chloride The molar ratio with carboxylate dye reaches 2:1, and the recovery rate reaches 91.7%.
Embodiment 2-1
[0061] Embodiment 2-1, a method for recovering alkali-washable disperse dye hydrolyzed dyes, the alkali washing solution obtained by alkali washing the cloth samples dyed with orange 1 dye in 0.1g / L NaOH aqueous solution; after detection and calculation, the In the alkaline washing solution, the molar concentration of the carboxylate dye as the hydrolysis product is 3.2mmol / L.
[0062] Carboxylate dyes formed after alkaline washing
[0063]
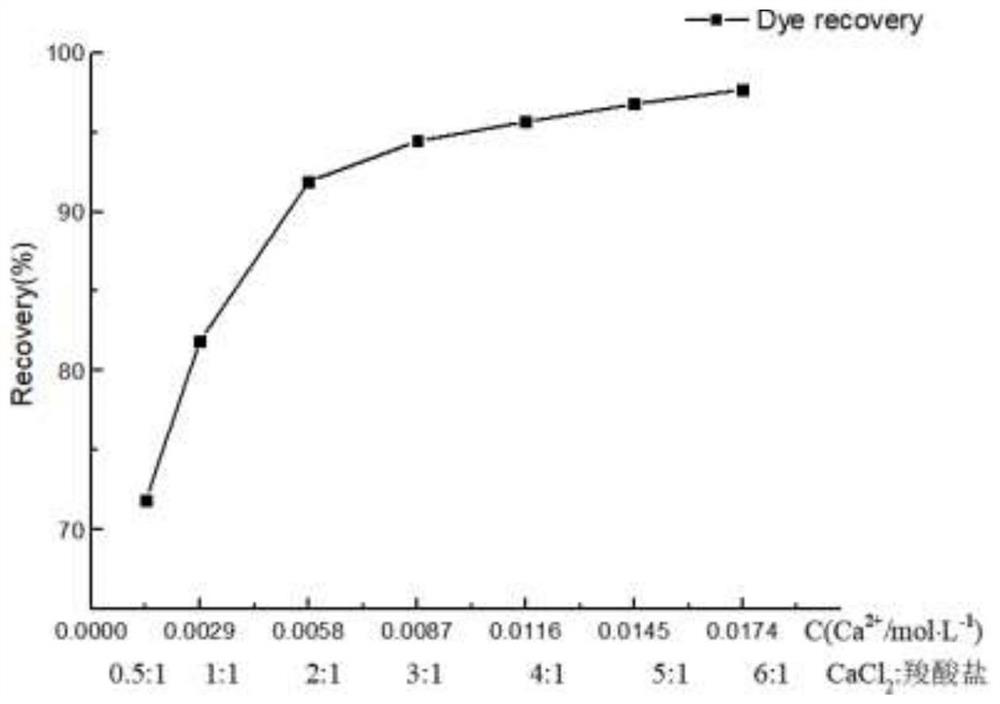
[0064] Add different amounts of calcium chloride saturated solution to the alkali washing solution, so that the molar ratio of calcium chloride: carboxylate dye is 0.5:1, 1:1, 2:1, 3:1, 4:1, 5:1, 6:1, after fully stirring for 1 hour, let stand for 24 hours. The recovery curve of carboxylate dye precipitation in different molar ratios, such as image 3 shown.
[0065] When the molar ratio of calcium chloride to carboxylate dye reaches 2:1, that is, when the calcium ion concentration is 6.4mmol / L, the recovery rate reaches 91.9%.
[...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com