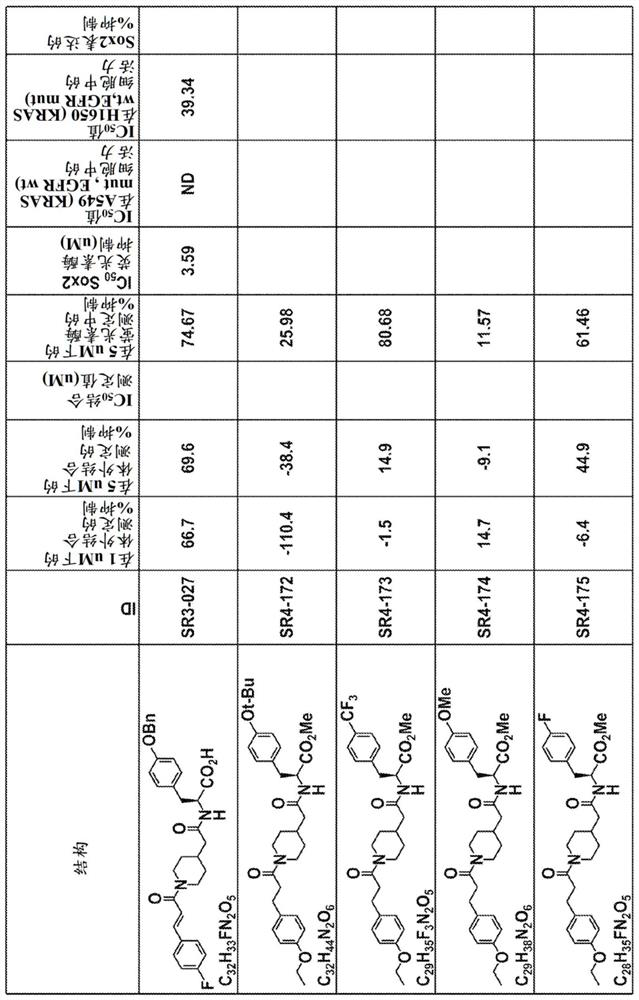
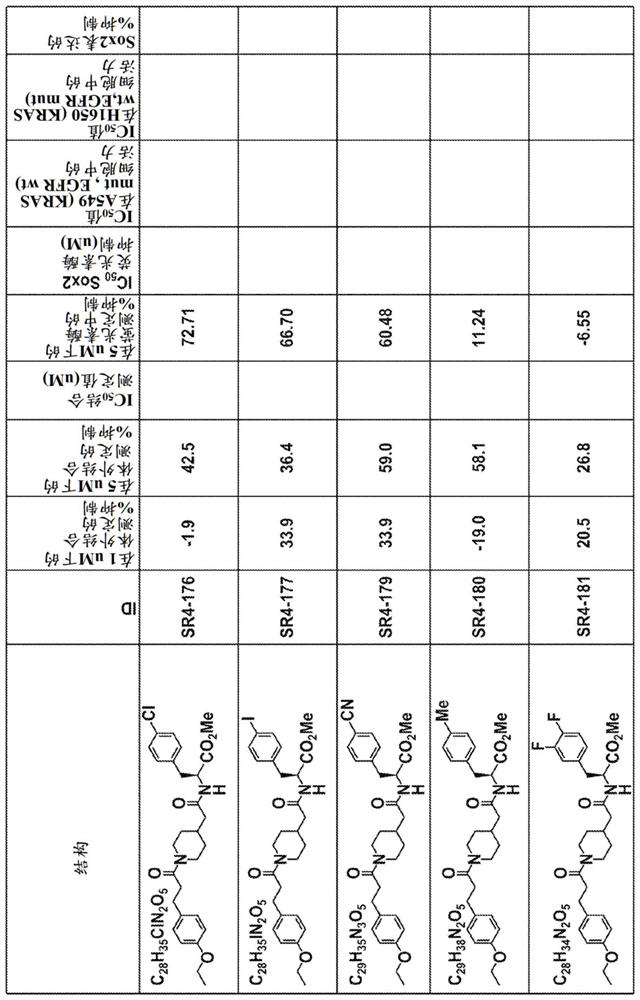
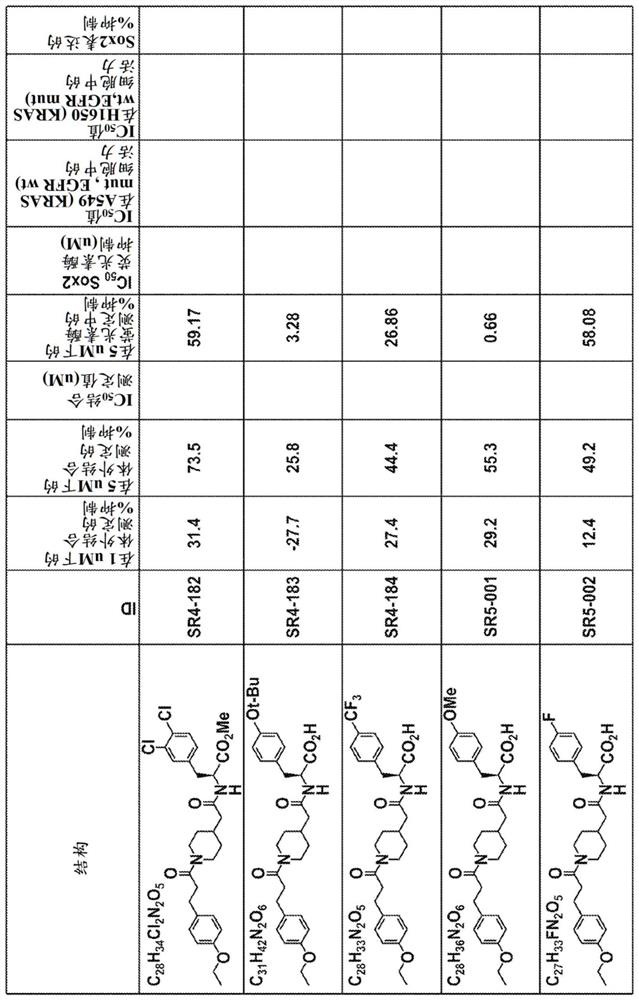
Yap1 inhibitors that target the interaction of yap1 with oct4
A C1-C6, C1-C8 technology, applied in the field of cancer treatment and anti-cancer compounds, YAP1 inhibitors, can solve problems such as low overall survival rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0147] Example 1. (S)-3-cyclohexyl-2-(2-(1-(3-(3,4-dichlorophenyl)propionyl)piperidin-4-yl)acetamido)propionic acid Methyl ester (SR3-137).
[0148] Using the general method, (S)-methyl 2-amino-3-cyclohexylpropanoate hydrochloride (0.019 g, 0.087 mmol) gave SR3-137 as a white foam (0.036 g, 95%). HPLC: >98%[t R = 5.0 min, 75% MeOH, 25% water (with 0.1% TFA), 20 min]. 1 H NMR (500MHz, DMSO-d 6 )δ8.18(d, J=7.9Hz, 1H), 7.54(bs, 1H), 7.52(d, J=8.2Hz, 1H), 7.25(dd, J=8.3, 2.1Hz, 1H), 4.37– 4.27(m,2H),3.89–3.77(m,1H),3.63–3.57(m,3H),3.03–2.83(m,2H),2.81(t,J=7.6Hz,2H),2.68–2.57( m,2H),2.05(d,J=7.1Hz,2H),1.94–1.82(m,1H),1.72–1.56(m,8H),1.55–1.44(m,2H),1.39–1.22(m, 2H), 1.22–1.05(m,3H), 1.04–0.76(m,4H). HRMS (ESI+): C 26 h 37 Cl 2 N 2 o 4 (M+H) + The calculated value of m / z is 511.2125, and the measured value is 511.2142; C 26 h 36 Cl 2 N 2 o 4 Na(M+Na) + m / z calculated value 533.1944, found value 533.1951; HPLC-MS (ESI+): m / z 533.2 [100%, (M+Na) + ].
[0149]
Embodiment 2
[0150] Example 2. (S)-4-methyl-2-(2-(1-(3-(3,4-dichlorophenyl)propionyl)piperidin-4-yl)acetamido)pentanoic acid Methyl ester (SR3-139).
[0151] Using the general method, SR3-139 was obtained from L-leucine methyl ester hydrochloride (0.016 g, 0.087 mmol) as a white foam (0.032 g, 94%). HPLC: >96%[t R = 5.2 min, 70% MeOH, 30% water (with 0.1% TFA), 20 min]. 1 H NMR (500MHz, DMSO-d 6 )δ8.18(d, J=7.6Hz, 1H), 7.54(d, J=2.1Hz, 1H), 7.52(d, J=8.2Hz, 1H), 7.25(dd, J=8.2, 2.1Hz, 1H),4.40–4.18(m,2H),3.84(m,1H),3.61(s,3H),3.02–2.88(m,1H),2.88–2.73(m,2H),2.68–2.58(m, 2H),2.56(m,1H),2.05(d,J=7.3Hz,2H),1.96–1.80(m,1H),1.69–1.50(m,4H),1.51–1.38(m,1H),1.09 -0.92 (m, 2H), 0.89 (d, J=6.6Hz, 3H), 0.84 (d, J=6.5Hz, 3H). HRMS (ESI+): C 23 h 33 Cl 2 N 2 o 4 (M+H) + The m / z calculated value of 471.1812, the measured value of 471.1826; C 23 h 32 Cl 2 N 2 o 4 Na(M+Na) + m / z calculated value 493.1631, found value 493.1646; HPLC-MS (ESI+): m / z 471.3 [80%, (M+H) + ], m / z 493.2 [100%...
Embodiment 3
[0153] Example 3. (S)-2-(2-(1-(3-(3,4-dichlorophenyl)propionyl)piperidin-4-yl)acetamido)-3-(4-fluoro Methyl phenyl)propionate (SR3-174).
[0154] SR3-174 was obtained as a white foam (0.034 g, 89 %). HPLC: >99%[t R = 5.1 min, 70% MeOH, 30% water (with 0.1% TFA), 20 min]. 1 H NMR (500MHz, DMSO-d 6 )δ8.27(d,J=8.0Hz,1H),7.53(bs,1H),7.51(m,1H),7.25(m,2H),7.10(dd,J=8.8,1.8Hz,2H), 4.50(m,1H),4.26(m,1H),3.76(m,1H),3.61(s,3H),3.04(dd,J=13.8,5.2Hz,1H),2.91–2.75(m,4H) ,2.61(m,2H),2.48–2.36(m,1H),1.97(d,J=7.2Hz,2H),1.75(m,1H),1.48(m,1H),1.35(m,1H), 0.99–0.70 (m,2H). HRMS (ESI+): C 26 h 30 Cl 2 FN 2 o 4 (M+H) + The calculated value of m / z is 523.1561, and the measured value is 523.1584; C 26 h 29 Cl 2 FN 2 o 4 Na(M+Na) + m / z calculated value 545.1381, found value 545.1493; HPLC-MS (ESI+): m / z 545.2 [100%, (M+Na) + ].
[0155]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com