Application of baicalein in preparation of medicine for preventing and treating obesity and complications thereof
A technology of baicalein and obesity, which is applied in the field of application of baicalein in the preparation of drugs for preventing and treating obesity and its complications, and can solve the problems of preventing and/or treating obesity and its complications by baicalein
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
experiment example 1
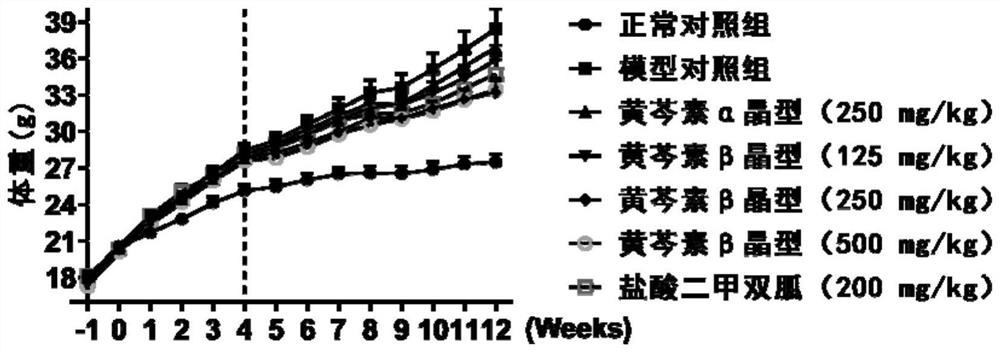
[0049] Experimental example 1. Effects of two crystal forms of baicalein on body weight and food intake of obese mice induced by high-fat diet
[0050] Experimental method: Changes in body weight, food intake and water intake of mice were recorded every week.
[0051] Experimental results: After being fed with high-fat diet, the weight of the mice gradually increased. After 4 weeks of feeding, the weight of the mice in the high-fat diet-induced obesity group increased significantly, which was statistically different from that of the normal control group. The two crystalline forms of baicalein were administered continuously for 8 weeks, gradually reducing the weight growth rate of obese mice induced by high-fat diet, and the anti-obesity biological activity of the β crystalline form was greater than that of the α crystalline form at the same dose (250 mg / kg) ( figure 1 ,Table 1). After 5 weeks of continuous administration of the two crystal forms of baicalein, the body weight ...
experiment example 2
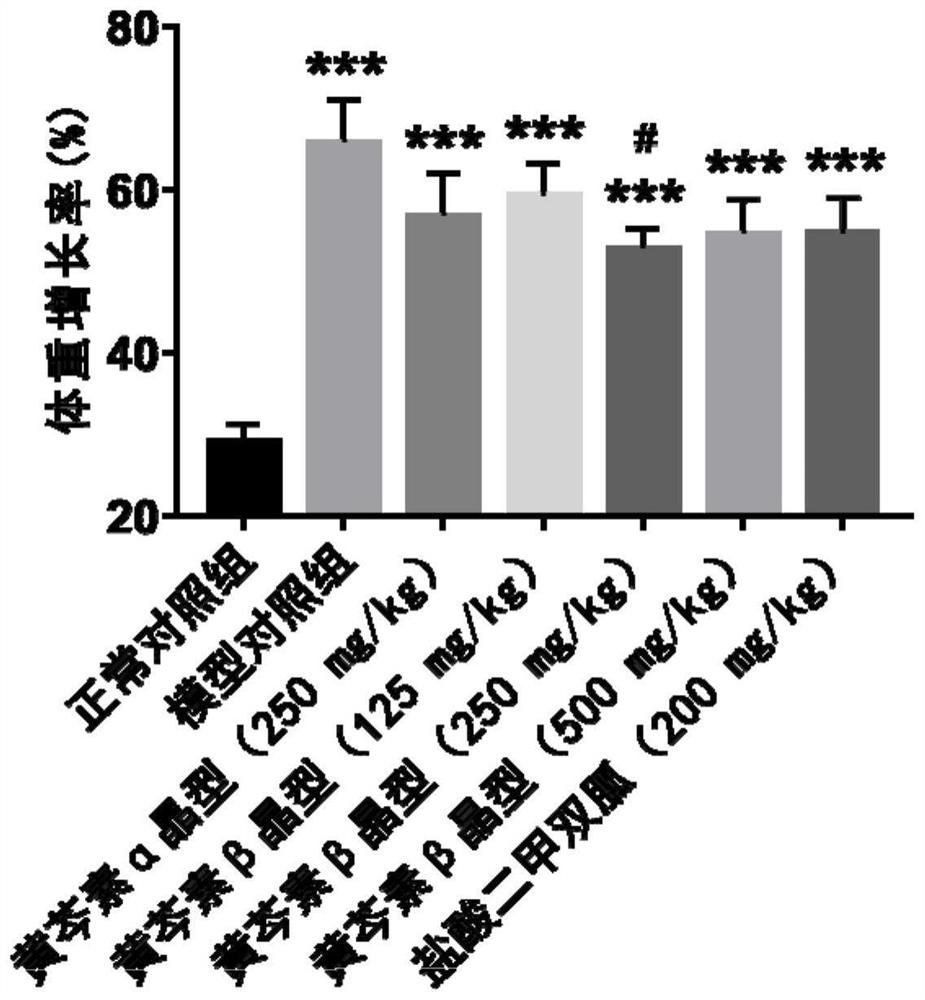
[0068] Experimental example 2. Effect of two crystal forms of baicalein on body fat rate of obese mice induced by high-fat diet
[0069] Experimental method: NMR method was used to detect the body fat percentage of mice. The higher the body fat percentage, the more serious the obesity of the mice. The body fat percentage decreased, indicating that the obesity status of the mice had improved.
[0070] Experimental results: Compared with the normal control group, the body fat rate of the model control group was significantly increased (P Figure 6 , Table 6).
[0071] Table 6 Effects of two crystal forms of baicalein on the body fat rate of obese mice induced by high-fat diet.
[0072]
[0073]Note: n=8-10, mean±SEM, t test was used for statistical analysis. ***P<0.001 (compared with normal control group), #P<0.05 (compared with model control group), ##P<0.01 (compared with model control group), ###P<0.001 (compared with model control group) compared to the model control gr...
experiment example 3
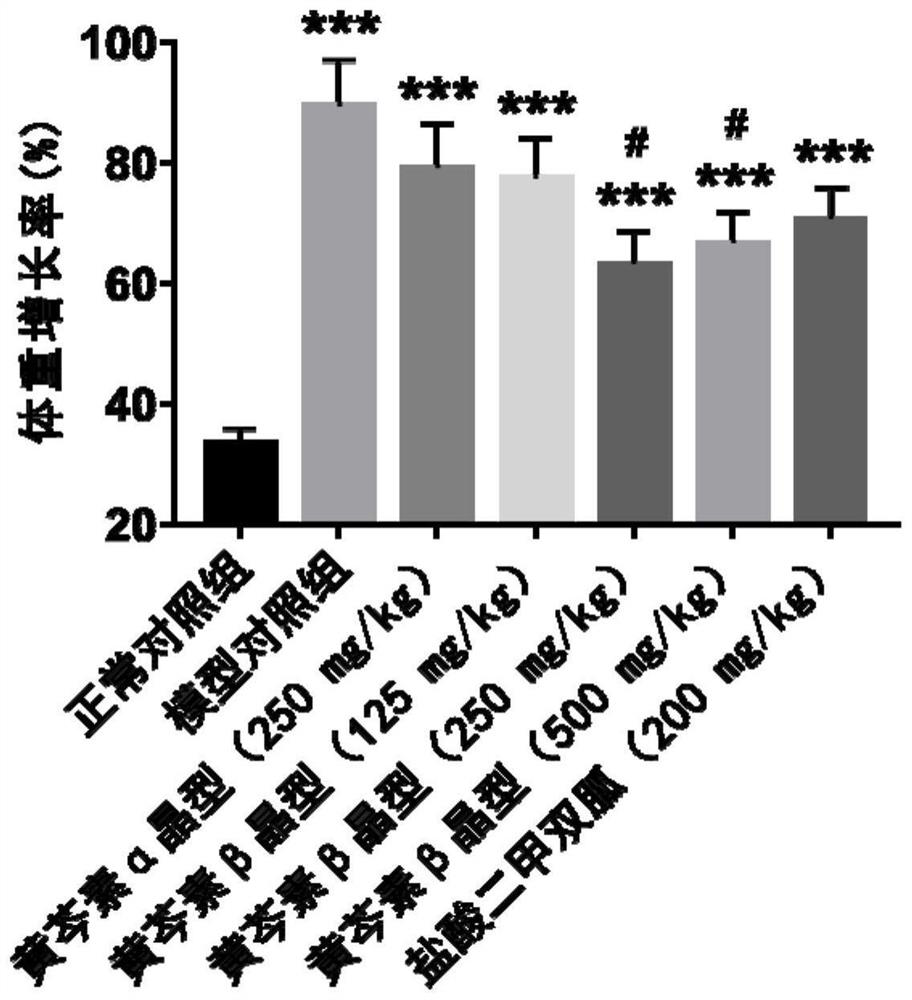
[0074] Experimental example 3. Effects of two crystal forms of baicalein on glucose metabolism in obese mice induced by high-fat diet
[0075] 3.1 Effects of two crystalline forms of baicalein administered for 4 weeks on fasting blood glucose in obese mice induced by high-fat diet
[0076] Experimental method: After 4 weeks of continuous administration of the two crystal forms of baicalein, the mice were fasted from 8:00 am, administered orally at 10:00, and blood was collected from the tip of the tail at 12:00. Determination of fasting blood glucose level in mice with Fulishan blood glucose test paper.
[0077] Experimental results: Compared with the normal control group, the fasting blood glucose of the mice in the model control group was significantly higher (P Figure 7 , Table 7).
[0078] Table 7 Effect of administration of two crystalline forms of baicalein for 4 weeks on fasting blood sugar in obese mice induced by high-fat diet.
[0079]
[0080] Note: n=8-10, mea...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com