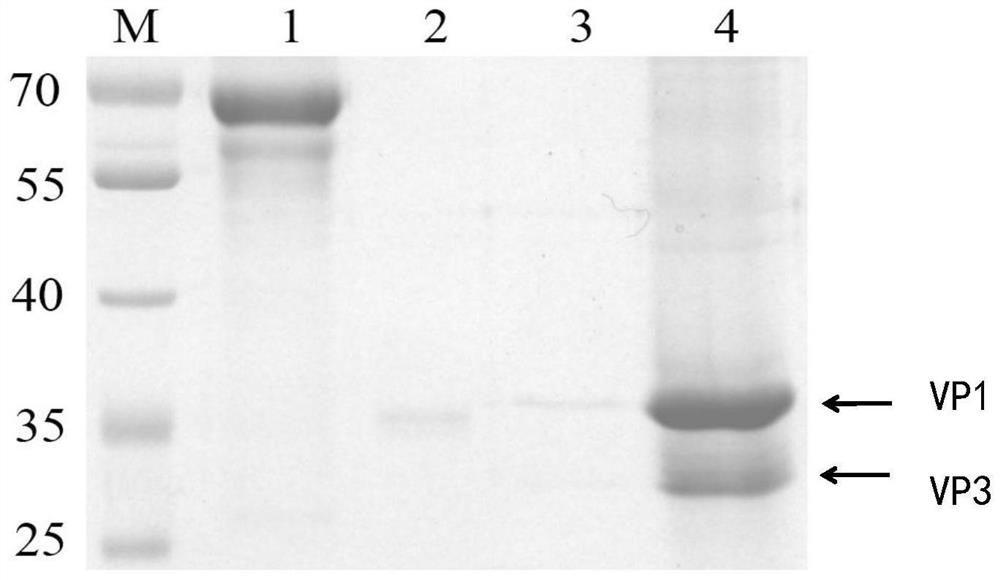
A kind of coxsackie virus b5 type virus-like particle, its preparation method and application
A coxsackie virus, virus-like technology, applied in the field of genetic engineering and biomedicine, can solve the problems of long production cycle, high production conditions, virus leakage and spread, etc., and achieve the goal of improving protection efficiency, strong immunity and long duration Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0074] The preparation method of Coxsackievirus B5 type virus-like particles of the present embodiment comprises the following steps:
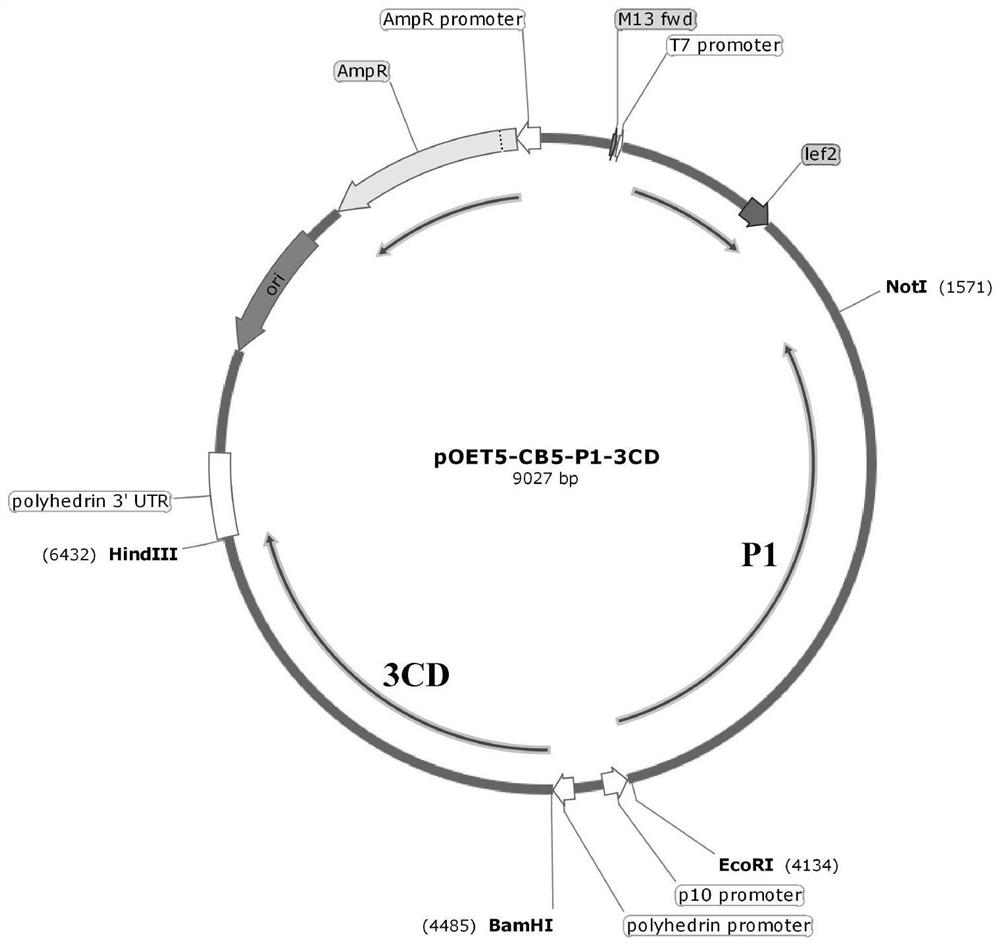
[0075] Step 1: Preparation of pOET5-CB5-P1-3CD recombinant plasmid
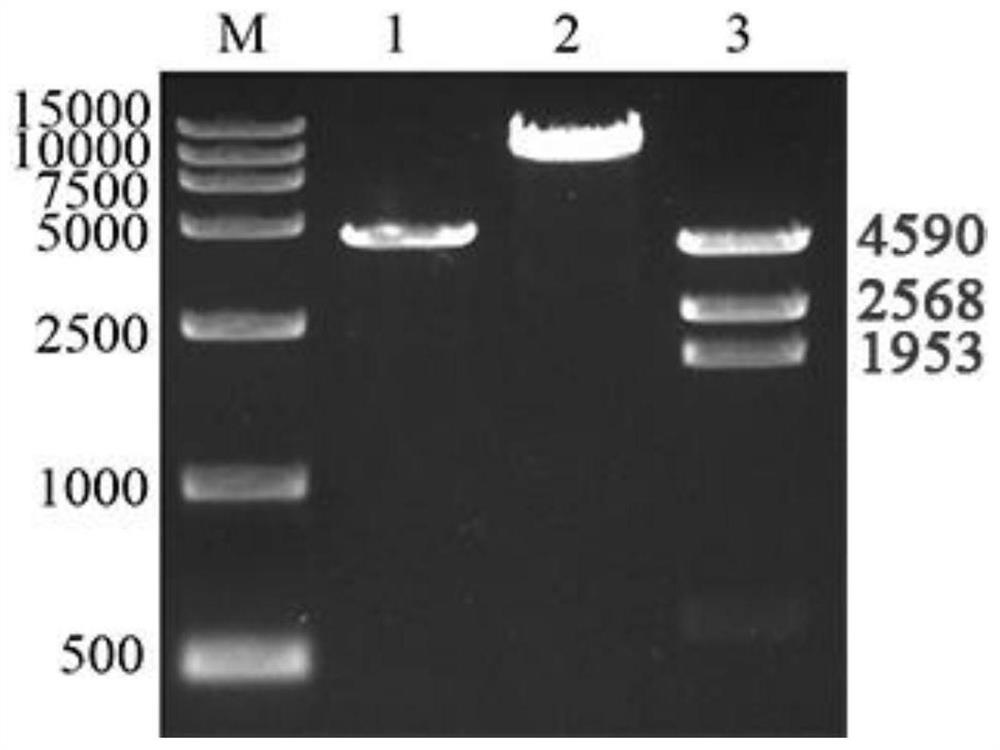
[0076] The codon-optimized Coxsackievirus B5 capsid protein P1 gene and 3CD protease gene were synthesized, and then respectively connected to the EcoRI / NotI restriction site and the P10 promoter of the insect baculovirus shuttle vector pOET5. The BamHI / Hind III restriction site behind the Ph promoter to obtain the pOET5-CB5-P1-3CD recombinant plasmid, such as figure 1shown. The main elements of the plasmid are as follows: p10 promoter is a P10 promoter, polyhedrin promoter is a Ph promoter, AmpR is an ampicillin resistance gene, and AmpR promoter is an ampicillin resistance gene promoter.
[0077] Wherein, the nucleotide sequence of the codon-optimized capsid protein P1 gene is shown in SEQ ID NO.1; the nucleotide sequence of the codon-optimized 3CD protease gene is shown ...
Embodiment 2
[0094] This embodiment provides a coxsackievirus type B5 virus-like particle vaccine, the coxsackievirus type B5 virus-like particle vaccine is composed of 50 μg / mL coxsackievirus type B5 virus-like particle obtained in Example 1 and Freud Equal volumes of adjuvant are mixed for liquid injection.
experiment example 1
[0095] Experimental Example 1: Animal Immunization and Serum Antibody Determination and Virus Neutralization Reaction
[0096] It was carried out according to the currently used animal immunization experiment method of enterovirus. The experimental animals were 6-week-old BALB / c female mice without specific pathogens (Specific pathogen Free, SPF). The specific operation is as follows: in the 0th week, the 2nd week and the 4th week, each BALB / c mouse was subcutaneously injected with 0.3ml of coxsackievirus B5 virus-like particles (10 μg total) emulsified in Freund's adjuvant. protein), with the same dose of inactivated Coxsackievirus B5 virus as the positive control, and the PBS group as the negative control, the mice were treated at the 0th week, the 2nd week, the 4th week, the 6th week and the 12th week Blood was collected by tail docking, and serum was taken for ELISA experiment and microneutralization experiment.
[0097] The steps of the ELISA experiment are as follows: ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com