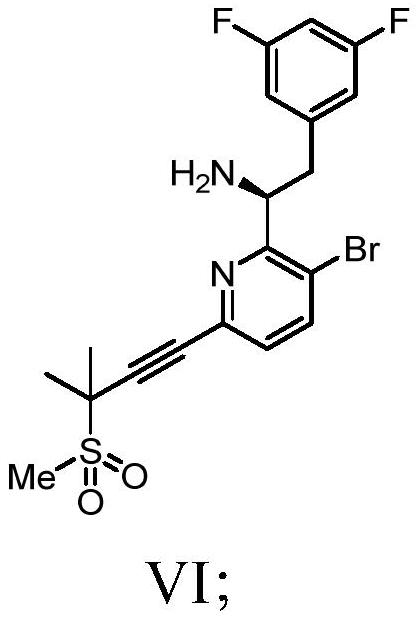
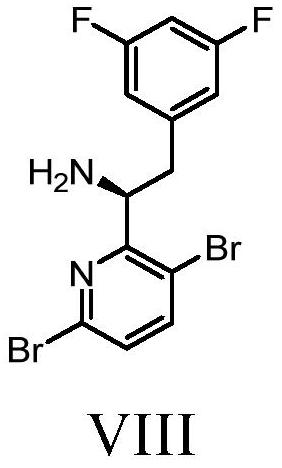
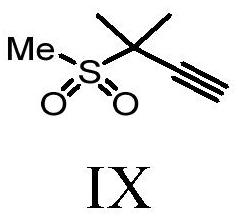Methods and intermediates for preparing a therapeutic compound useful in the treatment of retroviridae viral infection
A kind of compound and solvate technology, used in the field of preparation of therapeutic compounds and intermediates that can be used for the treatment of retroviral infection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1a
[1136] Example 1a: Preparation of (S)-1-(3,6-dibromopyridin-2-yl)-2-(3,5-difluorophenyl)ethan-1-amine (VIII-02) or its co- Crystals, solvates, salts or combinations, and starting materials and / or intermediates therein
[1137]
[1138] Synthesis of 3,6-Dibromopyridinecarbaldehyde (1a)
[1139]
[1140] 2,5-Dibromopyridine (1.0 g) was added to a dry reaction vial with a magnetic stir bar. The flask was inertized under nitrogen, THF (4.2 mL) was added, and the slurry was stirred. Separately, 2,2,6,6-tetramethylpiperidinylmagnesium chloride, lithium chloride complex (TMPMgCl·LiCl) (5.8ml, 6.3mmol) was added in a dry glass reactor. The TMPMgCl·LiCl solution was stirred and cooled to about -20°C. The 2,5-dibromopyridine solution was added to the TMPMgCl·LiCl solution over about 30 minutes, keeping the temperature below about -18°C. After the addition was complete, the flask was rinsed into the reactor with three additional portions of THF (1 mL x 2) and aged at about -2...
Embodiment 1b
[1168] Example 1b: For the formation of (S)-1-(3,6-dibromopyridin-2-yl)-2-(3,5-difluorophenyl)ethan-1-amine (VIII) or its co- Preparation of Alternative Starting Materials and Intermediates for Crystals, Solvates, Salts or Combinations
[1169] Synthesis of (R)-1-(3,6-dibromopyridin-2-yl)-2-(3,5-difluorophenyl)ethan-1-ol (XII)
[1170]
[1171] Compound XI (1.00 g) and (R)-RuCY-XylBINAP (16 mg, 0.05 equiv) were charged into a stainless steel autoclave fitted with a glass inner tube. EtOH (1.0 mL) and IPA (1.0 mL) were added to the autoclave, followed by tert-BuOK (1.0 M solution in THF, 0.51 mL, 0.2 equiv). with H 2 After purging, add 3MPa to the autoclave H 2 . The mixture was stirred at about 20°C for about 10 hours. Concentrated aqueous HCl was added to the mixture, and the pH was adjusted to 2. 1H NMR (400MHz, CDCl3): δ7.72(d, J=8.2Hz, 1H), 7.33(d, J=8.2Hz, 1H), 6.80–6.72(m, 2H), 6.68(tt, J=9.2 ,2.4 Hz,1H),5.16(dd,J=8.2,3.4Hz,1H),3.60(br,1H),3.12(dd,J=13.8,3....
Embodiment 1c
[1196] Example 1c: Racemization via (S)-1-(3,6-dibromopyridin-2-yl)-2-(3,5-difluorophenyl)ethan-1-amine (VIII) Preparation of 1-(3,6-dibromopyridin-2-yl)-2-(3,5-difluorophenyl)ethan-1-amine (X)
[1197]
[1198] A vial was charged with zinc acetate (25M%), enantiomerically enriched VIII (1.0 g, enantiomeric ratio 92:8), toluene (10 ml) and 2-formylpyridine (5 mol%). The vial was warmed to about 60°C and stirred for about 4 hours.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com