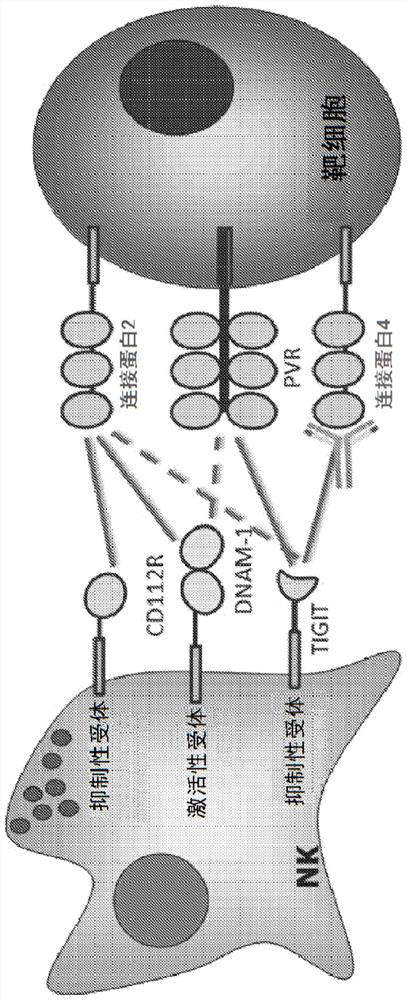
Antibodies specific to human nectin4
A connexin and antibody technology, applied in the direction of animal/human protein, anti-animal/human immunoglobulin, antibody, etc., can solve problems such as unmet needs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0376] Example 1. Generation and selection of anti-connexin-4 mAbs
[0377] The immunogen (Nec4-Fc) expression technology is based on mammalian HEK 293T cells and is a technology of choice giving the best quality, stability, solubility and yield especially in the case of glycoproteins. Nec4-Fc protein This fusion protein of the ectodomain of connexin-4 and the Fc domain of human IgG1 was recombinantly produced and purified as follows:
[0378] The coding sequence of human connexin 4 was cloned as a fusion with the Fc fragment of human IgG1 to generate a recombinant Fc-fusion protein. The ectodomain (extracellular) portion of the human connexin 4 molecule, which spans residues 32 to 349, was used. The C-terminal serine residue at position 349 of the connexin 4 amino acid sequence was fused to the heavy chain hinge of deglycosylated Fc (N297A) of human IgGl, followed by the CH2 and CH3 constant regions. The open reading frame (ORF) of the recombinant protein is codon-optimized...
Embodiment 2
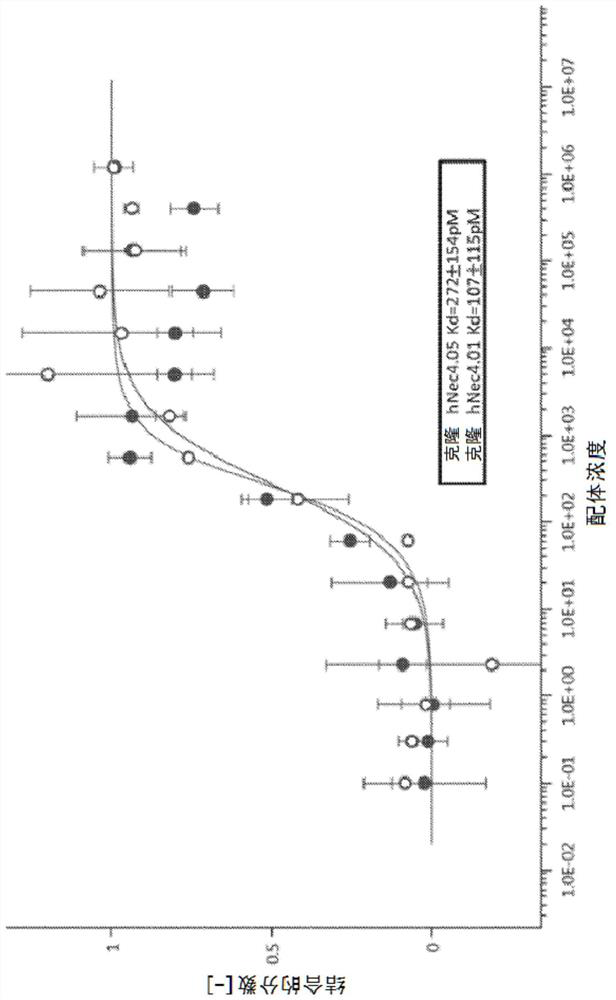
[0385] Example 2. Affinity of anti-connexin-4 mAbs for human connexin 4
[0386] The affinity of antibodies hNec4.01 and hNec4.05 to fluorophore-labeled human connexin 4-Ig molecules was further determined using a microthermophoresis assay (Wienken et al., 2010, Nat. Commun. 1:100). Measurements were repeated using at least three independent protein preparations. Such as image 3 As shown in , very high binding affinities were observed for both antibodies. The calculated Kd was 272±154 pM for clone hNec4.05 and 107±115 pM for the hNec4.01 clone.
Embodiment 3
[0387] Example 3. Sequencing of anti-connexin-4 mAbs
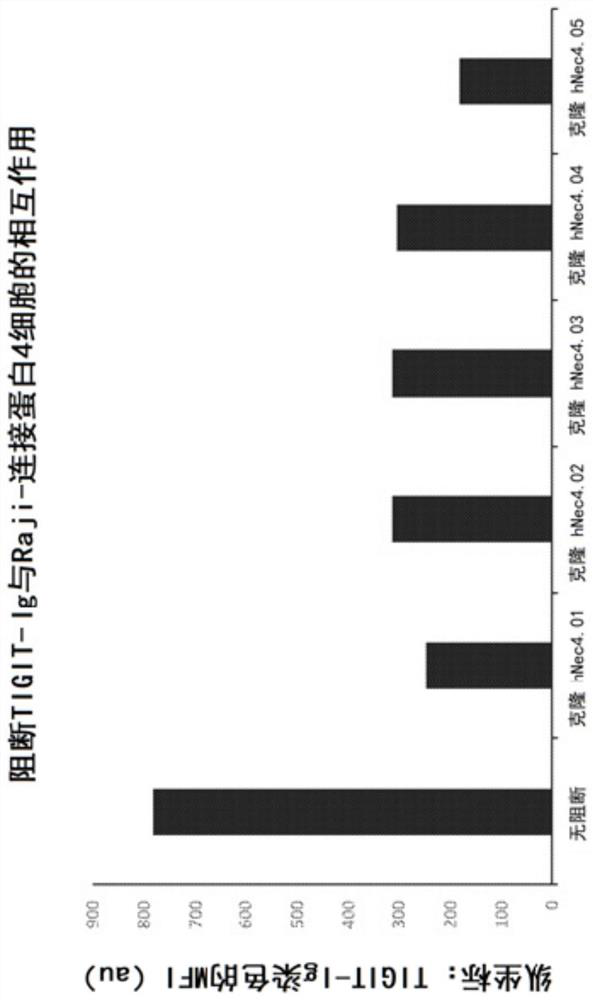
[0388] The two hybridoma clones No. 0.1 and No. 0.5 that demonstrated the best inhibition of Connexin 4-TIGIT binding were sent for nucleotide and amino acid sequencing.
[0389] method
[0390] comply with Reagents (Ambion, cat. no. 15596-026), total RNA was isolated from the hybridoma cells. Then follow PrimeScript TM Total RNA was reverse transcribed into cDNA using isotype-specific antisense primers or universal primers from the technical manual of the First Strand cDNA Synthesis Kit (Takara, cat#6110A). Amplify V according to GenScript's Rapid Amplification of cDNA Ends (RACE) standard operating procedure (SOP) H and V Lantibody fragments. The amplified antibody fragments are cloned individually into standard cloning vectors. Colony PCR was performed to screen for clones with inserts of the correct size. For each fragment, no fewer than 5 clones with inserts of the correct size were sequenced. The sequences ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com