IRAK4 kinase inhibitor and preparation method thereof
A technology of solvate and alkyl, applied in the field of IRAK4 kinase inhibitor and its preparation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
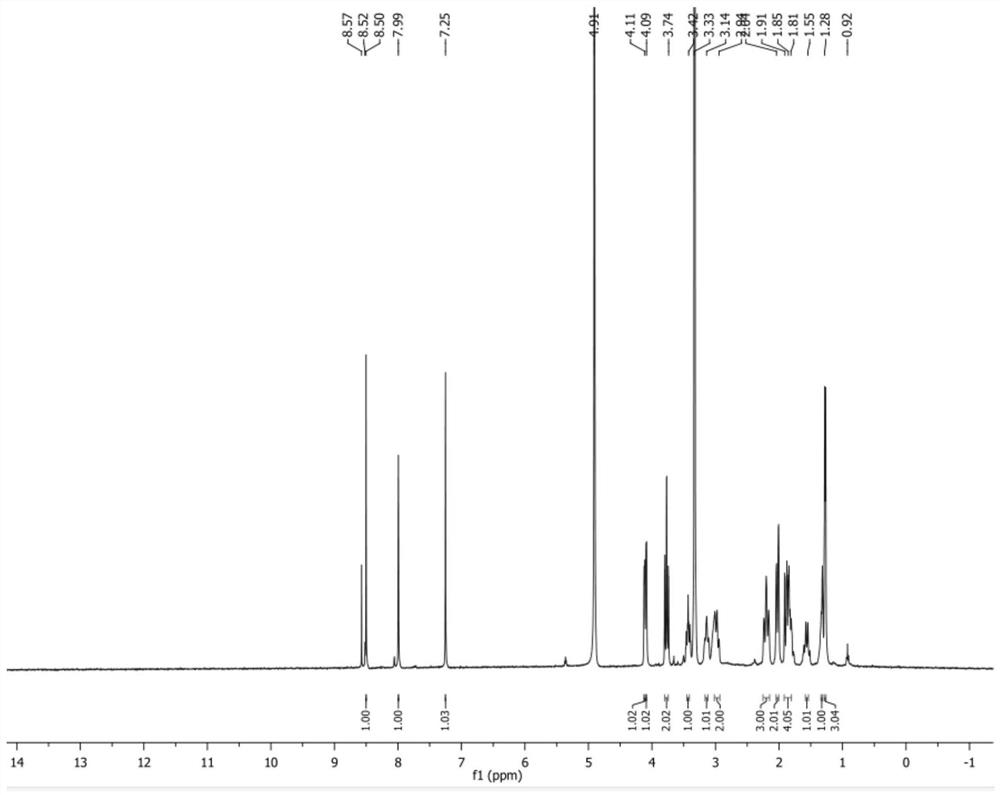
[0115] The preparation of embodiment 1IRAK4 kinase inhibitor
[0116] The synthetic route is as follows:
[0117]
[0118] step 1:
[0119]
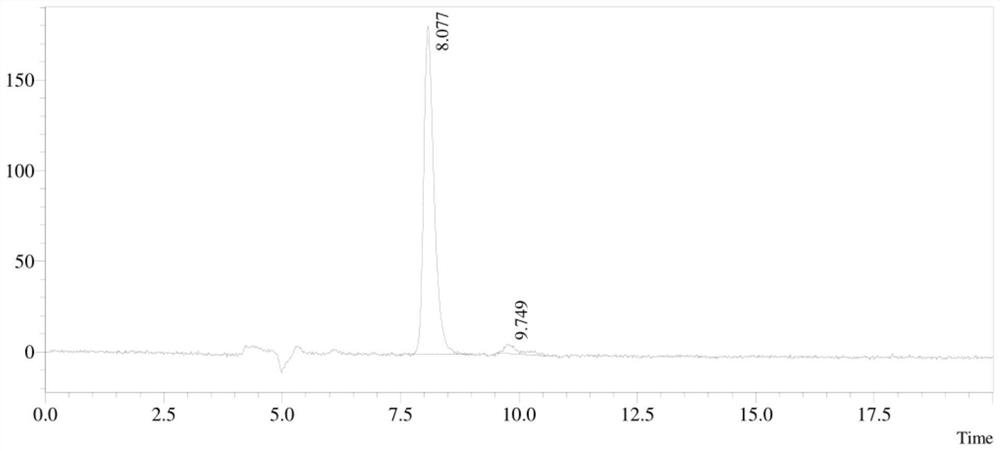
[0120] Add 1 (8.0g, 40.62mmol), dichloromethane (80mL), triethylamine (8.23g, 81.23mmol) and TsCl (9.29g, 48.74mmol) in a 250ml one-necked bottle, stir at room temperature for 3 hours, add water, extracted with ethyl acetate (100mLx 3), washed with saturated brine (50mL x 2), dried over anhydrous sodium sulfate, spin-dried, and obtained the target product by column chromatography (petroleum ether: ethyl acetate = 4:1) As a yellow solid (8.0g, yield: 56.1%), LC-MS: 352[M+H] + .
[0121] Step 2:
[0122]
[0123] Add 2 (8.0g, 22.79mmol), tert-butyl carbamate (3.20g, 27.35mmol), DIEA (5.88g, 45.58mmol) and DMF (40.0mL) in a 100mL single-necked round bottom flask, at 100°C Reaction 18h. After adding water, it was extracted with ethyl acetate (100mL x 3), the organic phase was washed with saturated brine (50mL x 2), dried over ...
Embodiment 2
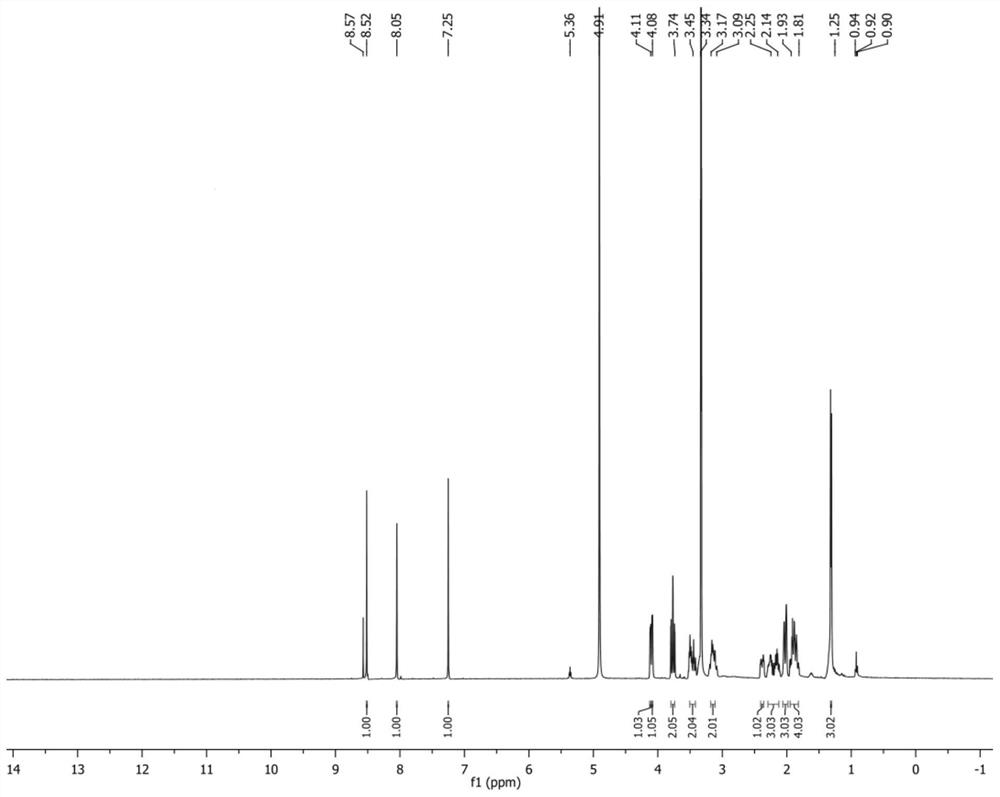
[0152] Preparation of embodiment 2 IRAK4 kinase inhibitor
[0153] The synthetic route is as follows:
[0154]
[0155] step 1:
[0156]
[0157] Add 1 (8.0g, 40.62mmol), dichloromethane (80mL), triethylamine (8.23g, 81.23mmol) and TsCl (9.29g, 48.74mmol) in a 250ml one-necked bottle, stir at room temperature for 3 hours, add water, extracted with ethyl acetate (100mLx 3), washed with saturated brine (50mL x 2), dried over anhydrous sodium sulfate, spin-dried, and obtained the target product by column chromatography (petroleum ether: ethyl acetate = 4:1) As a yellow solid (8.0g, yield: 56.1%), LC-MS: 352[M+H] + .
[0158] Step 2:
[0159]
[0160] Add 2 (8.0g, 22.79mmol), tert-butyl carbamate (3.20g, 27.35mmol), DIEA (5.88g, 45.58mmol) and DMF (40.0mL) in a 100mL single-necked round bottom flask, at 100°C Reaction 18h. After adding water, it was extracted with ethyl acetate (100mL x 3), the organic phase was washed with saturated brine (50mL x 2), dried over anh...
Embodiment 3
[0187] The preparation of embodiment 3IRAK4 kinase inhibitor
[0188] The synthetic route is as follows:
[0189]
[0190] step 1:
[0191]
[0192] Add 1 (8.0g, 40.62mmol), dichloromethane (80mL), triethylamine (8.23g, 81.23mmol) and TsCl (9.29g, 48.74mmol) in a 250ml one-necked bottle, stir at room temperature for 3 hours, add water, extracted with ethyl acetate (100mLx 3), washed with saturated brine (50mL x 2), dried over anhydrous sodium sulfate, spin-dried, and obtained the target product by column chromatography (petroleum ether: ethyl acetate = 4:1) As a yellow solid (8.0g, yield: 56.1%), LC-MS: 352[M+H] + .
[0193] Step 2:
[0194]
[0195] Add 2 (8.0g, 22.79mmol), tert-butyl carbamate (3.20g, 27.35mmol), DIEA (5.88g, 45.58mmol) and DMF (40.0mL) in a 100mL single-necked round bottom flask, at 100°C Reaction 18h. After adding water, it was extracted with ethyl acetate (100mL x 3), the organic phase was washed with saturated brine (50mL x 2), dried over ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com