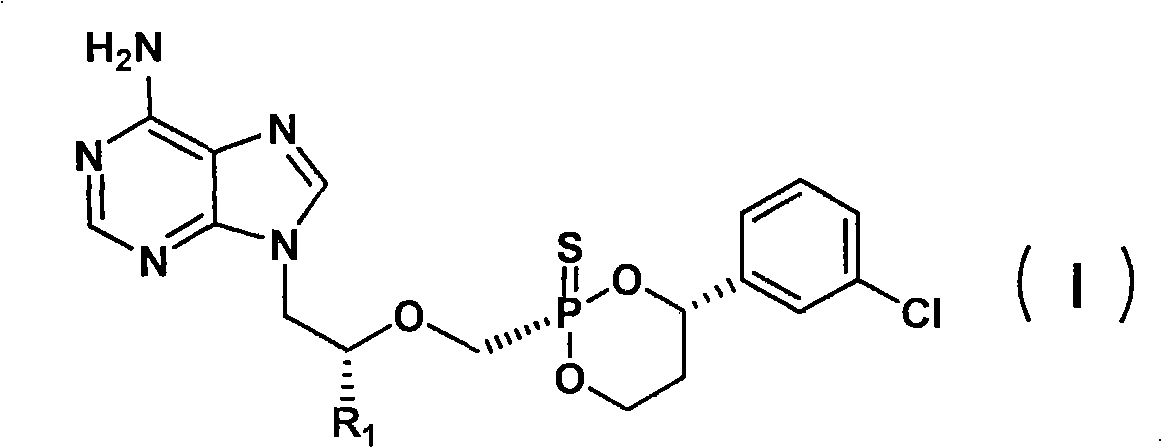
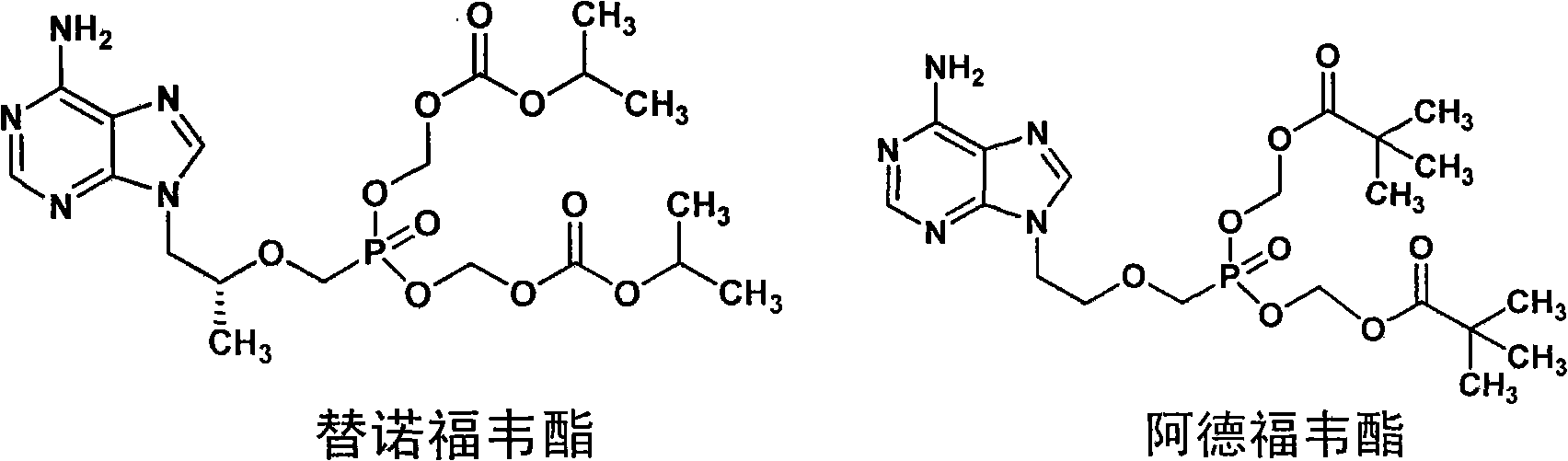
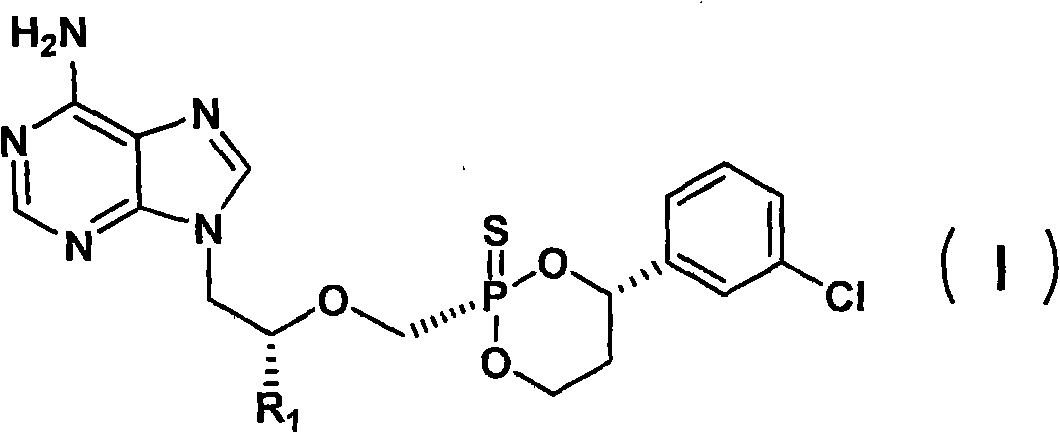
Sulfo-Adefovir and Tenofovir liver targeting ester prodrug
A prodrug, tenofovir technology, applied in the field of prodrugs, can solve problems such as inability to transport drugs, toxicity, lack of effective therapeutic drugs, etc., and achieve simple and easy production method, good oral absorption, and good market. Foreground effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example 1
[0030] Preparation Example 1: Preparation of sulfur instead of Nofovir
[0031] 1.1. Preparation of (R)-1,2-propanediol
[0032] The air in the hydrogenation kettle was replaced with N 2 Charge N after replacement 2 , add 5% Pd loaded on activated carbon (50% moisture content) catalyst 100g suspended in NaOH ethanol solution (composed of 7.85Kg ethanol and 16.7% NaOH solution 54g), stir and cool to 0 ° C (usually -5 ~ 0 ° C About), then add (S)-glycidol 1.0Kg (13.5mol), and then use H2 Replacement of N in the kettle 2 3 times, charge H 2 (pressure ≤ 13.79KPa or ≤ 20psi) reaction, the reaction temperature is controlled not to exceed 25°C. Charge H 2 The reaction ends when the hydrogen is no longer consumed. After the reaction was completed, the reaction solution was filtered through diatomaceous earth (150g) to remove solid insoluble matter, and the filtrate was evaporated in vacuo at 50°C to remove the solvent to obtain about 1.0Kg of oily R)-1,2-propanediol crude produc...
preparation example 2
[0042] Preparation Example 2: Preparation of Thioadefovir
[0043] Referring to the method of Examples 1.4-1.6, adefovir was prepared by substituting commercially available ethylene carbonate for (R)-1,2-propylene carbonate.
preparation example 3
[0044] Preparation Example 3: Preparation of (S)-(-)-1-(3-chlorophenyl)-1,3-propanediol
[0045] 3.1, Preparation of 3-(3-chlorophenyl)-3-oxo-propionic acid
[0046] Install a mechanical stirrer and an addition funnel in a dry and clean 5L three-neck round bottom flask. Nitrogen was blown to drive away the air, diisopropylamine (424ml) and THF (1.2L) were added thereto, the stirred contents were cooled to -30°C, and n-butyllithium (1.23L, 2.5 M in hexane), and maintain the temperature at -30 to -48°C. After the addition was complete (30 minutes), the addition funnel was rinsed with hexane (30 ml), then the stirred solution was cooled to -70° C., trimethylsilylethyl acetate (200 g) was added slowly under stirring, and the temperature Keeping at 4 , 100 g), filtered and concentrated under reduced pressure to give about 503 g of a yellow solid, the crude solid was slurried in hexane (3.5 L) and transferred to a round bottom flask equipped with a mechanical stirrer, the mixture ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com