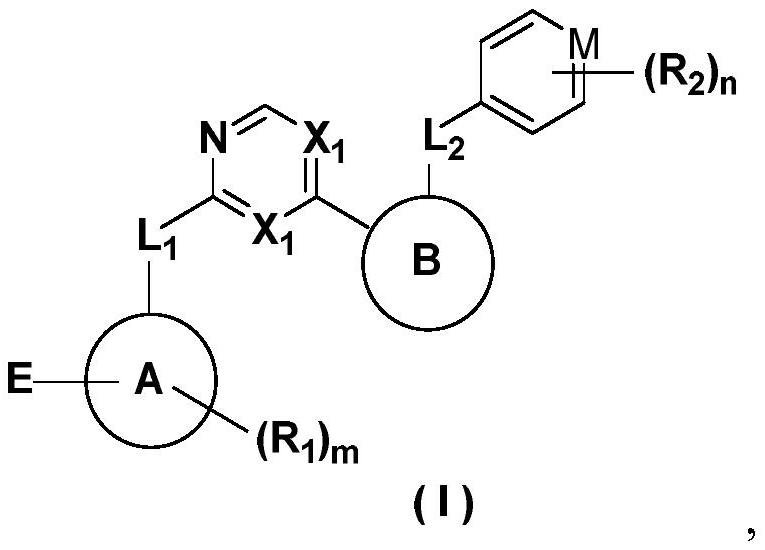
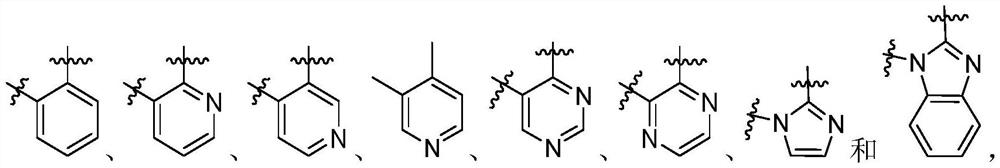
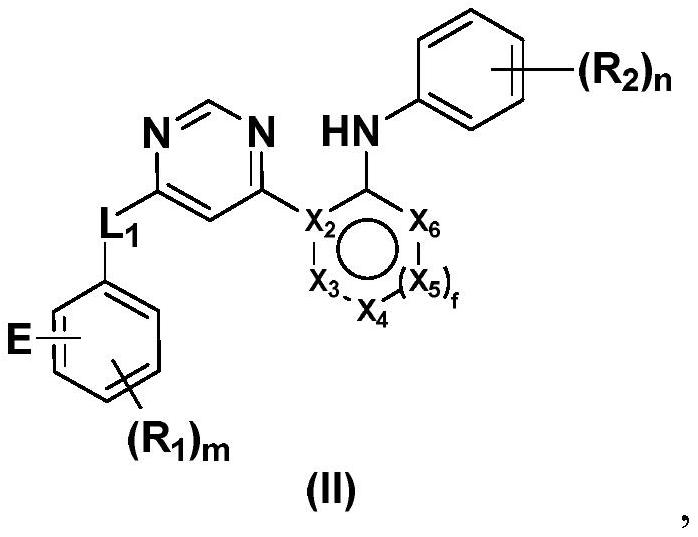
Heterocyclic compounds as FGFR4 inhibitors
A kind of technology of compound and solvate, applied in the field of treatment of cell proliferative diseases
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0128] Compound 1A:
[0129]
[0130] 2-Nitroaniline (5.0 g, 36.2 mmol) was dissolved in tetrahydrofuran (10.0 mL) at room temperature. Subsequently, sodium hydride (1.8 g, 43.5 mmol, 60% dispersion in mineral oil) was added portionwise to the above solution under an ice-water bath. After the reaction solution was stirred at room temperature for 30 minutes, the above reaction solution was slowly added to a THF solution (100.0 mL) of 4,6-dichloropyrimidine (10.8 g, 73.0 mmol). The reaction was continued to stir overnight at room temperature.
[0131] After LCMS monitoring showed that the starting material disappeared, water was added to the reaction system to quench it. The mixture was extracted with ethyl acetate, and the organic phases were combined. The organic phase was first washed with saturated brine, then dried over anhydrous sodium sulfate, filtered, and finally concentrated under reduced pressure. The obtained residue was purified by silica gel column chromatogr...
Embodiment 2
[0166] Compound 2A:
[0167]
[0168] Compound 1A (420 mg, 1.68 mmol) was dissolved in a mixed solution of 1,4-dioxane and water (12.0 ml, V / V=5 / 1) at room temperature under nitrogen protection. Subsequently, 2,5-difluoro-3-pyridineboronic acid (400 mg, 2.52 mmol), Pd(PPh 3 ) 4 (485 mg, 0.42 mmol) and anhydrous potassium carbonate (463 mg, 3.35 mmol). The reaction solution was heated to 80°C and stirred.
[0169] After LCMS monitoring showed that the starting material disappeared, the reaction solution was concentrated under reduced pressure. The resulting residue was purified by silica gel column chromatography to obtain compound 2A, 6-(2,5-difluoropyridin-3-yl)-N-(2-nitrophenyl)pyrimidine-4amino.
[0170] MS(ESI)M / Z:330.1[M+H + ].
[0171] Compound 2B:
[0172]
[0173] Compound 2A (160 mg, 0.48 mmol) and Compound 1C (215 mg, 0.97 mmol) were dissolved in toluene (10.0 mL) at room temperature under nitrogen protection. Subsequently, lithium bistrimethylsilylamide ...
Embodiment 3
[0188] Compound 3A:
[0189]
[0190] At room temperature under nitrogen protection, 4-chloro-6-methylthiopyrimidine (15.0 g, 93.8 mmol) was dissolved in a mixed solution of 1,4-dioxane and water (360 ml, V / V=5 / 1) in. Subsequently, 2-fluoro-3-pyridineboronic acid (26.4 g, 187 mmol), Pd(PPh 3 ) 4 (5.42 g, 4.69 mmol) and anhydrous potassium carbonate (25.9 g, 187 mmol). The reaction system was heated to 80°C and stirred.
[0191] After LCMS monitoring showed that the starting material disappeared, water was added to the reaction solution to quench it. The mixture was extracted with ethyl acetate, and the organic phases were combined, then dried over anhydrous sodium sulfate, filtered, and finally concentrated under reduced pressure. The obtained residue was purified by silica gel column chromatography to obtain compound 3A, 4-(2-fluoropyridin-3-yl)-6-(methylthio)pyrimidine.
[0192] MS(ESI)M / Z:222.2[M+H + ].
[0193] Compound 3B:
[0194]
[0195] Compound 3A (15...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com