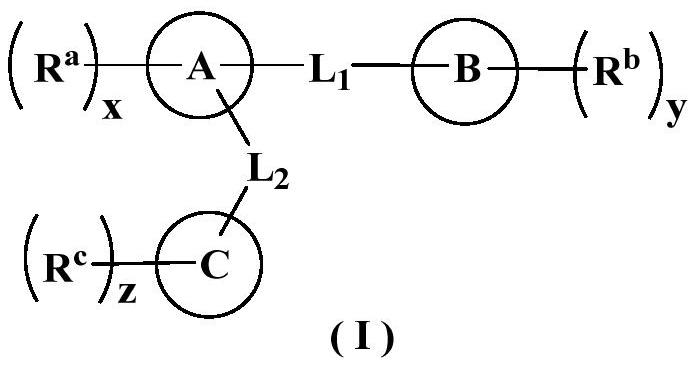
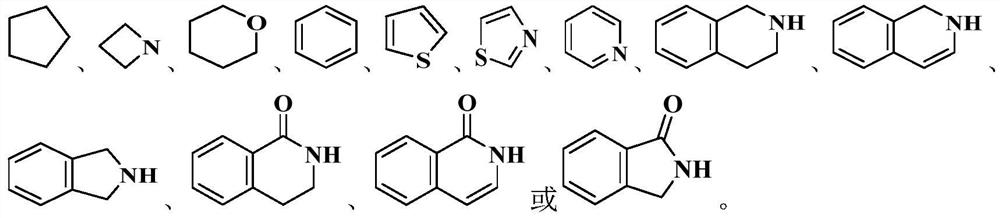
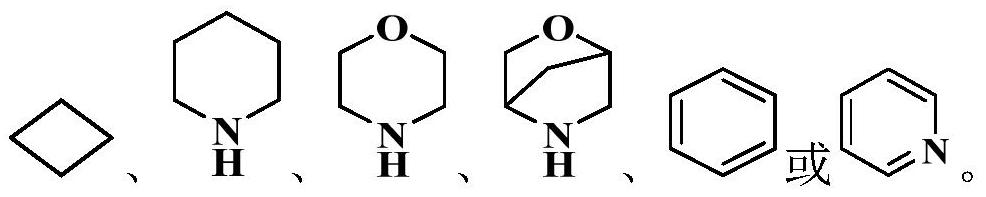
Heterocyclic derivative inhibitor as well as preparation method and application thereof
A technology of heterocyclyl and heterocyclylalkyl, which is applied in the field of heterocyclic derivative inhibitors and their preparation, and can solve problems such as loss of taste
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0190] 4-(1-(2-((5-chloropyridin-2-yl)amino)-2-oxoethyl)-5-methyl-1H-benzo[d]imidazol-2-yl)-3 -Fluoro-N-methylbenzamide
[0191]
[0192] The first step: the preparation of 2-bromo-N-(5-fluoropyridin-2-yl)acetamide
[0193]
[0194] To a solution of (1 g, 7.78 mmol) 5-fluoro-pyridin-2-ylamine and 0.3 mL of pyridine in 20 mL of toluene was added dropwise (1.9 g, 7.92 mmol) bromoacetyl bromide dissolved in 5 mL of toluene under ice cooling . After 2 hours, a white solid was precipitated, and the precipitate was separated by filtration and recrystallized from toluene to obtain Example 1-1 (1.8 g, yield: 70%) as a white solid.
[0195] MS m / z(ESI):249.23[M+H] + .
[0196] 1 H NMR (400MHz, MeOD) δ8.29(d, J=2.7Hz, 1H), 8.12(d, J=8.9Hz, 1H), 7.80(dd, J=8.9, 2.7Hz, 1H), 4.04(s ,2H).
[0197] The second step: the preparation of 4-(bromomethyl)-3-fluorobenzoic acid
[0198]
[0199] 3-Fluoro-4-methyl-benzoic acid (7 g, 45.4 mmol) and NBS (8.08 g, 45.4 mmol) were dissolv...
Embodiment 2
[0214] (S)-6-(3-((4-acetylmorpholin-2-yl)methyl)-7-methylimidazol[1,2-a]pyridin-2-yl)-5,7-difluoro -2-Methyl-3,4-dihydroisoquinolin-1(2H)-one
[0215]
[0216] Step 1: Preparation of 5,7-difluoro-2-methyl-3,4-dihydroisoquinolin-1(2H)-one
[0217]
[0218] Example 2-1 (800mg, 5mmol) was dissolved in acetonitrile (10ml), and 1-chloromethyl-4-fluoro-1,4-diazobicyclo[2.2.2]octane bis(tetrafluoroboron Acid acid) (3.54g, 10mmol), the mixture was heated at 80°C to reflux overnight. The reaction solution was cooled to room temperature, concentrated and purified by silica gel column to obtain Example 2-2 (460 mg, 47% yield).
[0219] MS m / z(ESI):198.0[M+H] + ;
[0220] The second step: the preparation of 5,7-difluoro-2-methyl-1-oxo-1,2,3,4-tetrahydroquinoline-6-carbaldehyde
[0221]
[0222] To a solution of Example 2-2 (460 mg, 2.33 mmol) in 2-methyltetrahydrofuran (6 mL) was added TMEDA (595 mg, 5.13 mmol) at room temperature. The resulting solution was cooled to -78°C...
Embodiment 3
[0229] (S)-6-(3-((4-acetylmorpholin-2-yl)methyl)-7-methylimidazol[1,2-a]pyridin-2-yl)-5,7-difluoro -2-Methylisoquinolin-1(2H)-one
[0230]
[0231] Referring to Example 2 for the synthesis method of Example 3, 2-methylisoquinolin-1(2H)-one was used instead of Example 1-1 to obtain Example 3 (23 mg, 10% yield).
[0232] MS m / z(ESI):467.1[M+H] + .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com