Process for the preparation of quinmerac
A technology of quinolincarboxylic acid and chlorobenzonitrile, which is applied in botany equipment and methods, organic chemistry, animal husbandry, etc., and can solve problems such as low yield and waste
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0024] Preparation of quinclorac:
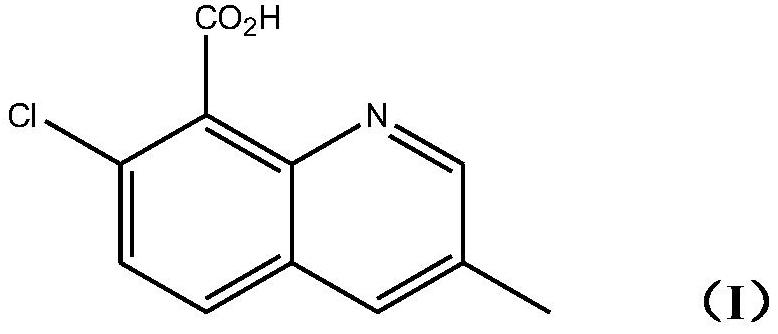
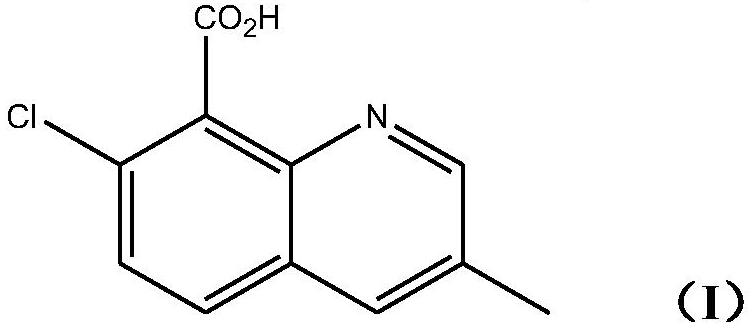
[0025] The invention provides a kind of method preparing the quinclorac represented by following formula (I):
[0026]
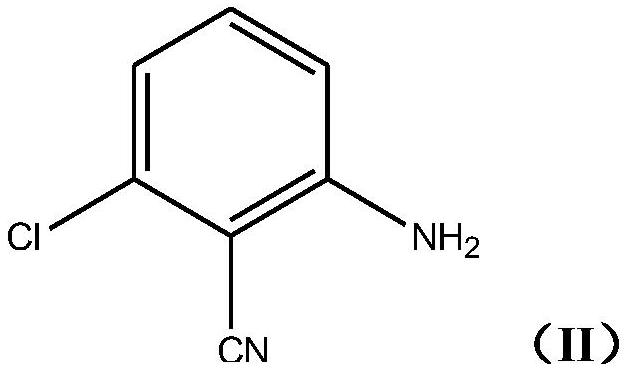
[0027] The method comprises making 2-amino-6-chlorobenzonitrile represented by the following formula (II):
[0028]
[0029] Reacts with methacrolein in the presence of acids and oxidizing agents.
[0030] In one embodiment of the invention, said acid is selected from sulfuric acid and hydrochloric acid.
[0031] In a specific embodiment, the acid is sulfuric acid.
[0032] In one embodiment of the method of the invention, the molar ratio between the compound of formula (II) and the acid is from about 1:1 to about 1:50. In another embodiment, the molar ratio between the compound of formula (II) and the acid is from about 1:1 to about 1:25.
[0033] In a particular embodiment, the molar ratio between the compound of formula (II) and the acid is about 1:10.
[0034] In another specific embodiment, the molar rati...
Embodiment
[0066]Raw material consumption on 2.0g scale
[0067] name mw equivalent g / batch (ml / batch) 2-Amino-6-chlorobenzonitrile 152.58 1.0 2.00g methacrolein 70.09 2.0 1.84g boric acid 61.83 1.0 0.81g Concentrated H 2 SO 4
98.08 10.0 12.86g FeSO 4 ·7H 2 o
278.01 0.2 0.73g Nitrobenzene 123.11 2.0 2.70ml water - - 4.80ml
[0068] All reagents including 2-amino-6-chlorobenzonitrile were purchased from Sigma-Aldrich.
[0069] general method
[0070] 2.00g (13.1mmol) 2-amino-6-chlorobenzonitrile, 0.81g (13.1mmol) boric acid, 12.86g (131.1mmol) concentrated H 2 SO 4 , 0.73g (2.6mmol) FeSO 4 ·7H 2 O. Add 2.16ml (1.84g; 26.2mmol) methacrolein dropwise to a suspension of 2.70ml (26.2mmol) nitrobenzene and 4.80ml water. The reaction mixture was then stirred at 130°C for 6 hours. The reaction mixture was cooled and poured into 50 g of ice water. The precipitate was filtered off, and the pH of the filt...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com