Methods and systems for sparse vector-based matrix transformations
A sparse vector and matrix technology, applied in biological systems, genomics, biostatistics, etc., can solve problems such as difficult query or transformation of data, lack of data, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0249] Embodiment 1. A method comprising:
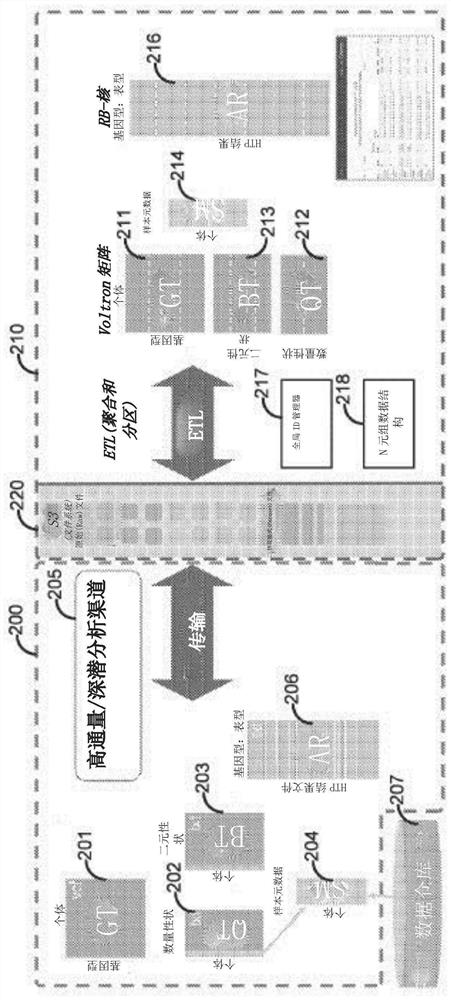
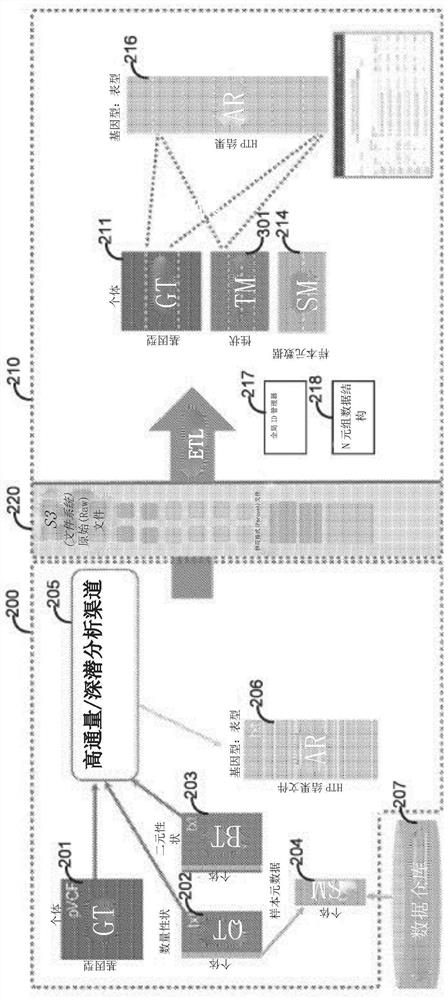
[0250] receiving genotype data and phenotype data for a plurality of individuals from a plurality of cohorts;
[0251] generating a genotype matrix based on the genotype data, wherein the genotype matrix includes a column for each of the plurality of individuals and a plurality of rows for each of the plurality of variants;
[0252] generating a quantitative trait matrix based on the phenotypic data, wherein the quantitative trait matrix includes a column for each of a plurality of quantitative traits and a plurality of rows for each of the plurality of individuals;
[0253] generating a binary trait matrix based on the phenotype data, wherein the binary trait matrix includes a column for each of a plurality of binary traits and a plurality of rows for each of the plurality of individuals;
[0254] appending at least a portion of a metadata matrix to each of the genotype matrix, the quantitative trait matrix, and the binary trait ma...
Embodiment 2
[0262] Embodiment 2. The method of embodiment 1, wherein the sparse vector representing one or more values of the genotype matrix comprises A data structure that associates columns for each group identifier.
Embodiment 3
[0263] Embodiment 3. The method of embodiment 1, wherein the sparse vector representing one or more values of the genotype matrix comprises a homozygous reference.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com