Methods and compositions for treating coronavirus infections
A virus infection and composition technology, applied in the direction of antiviral agents, active ingredients of hydroxyl compounds, drug delivery, etc., can solve the problems of evaluation, no virus, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 15
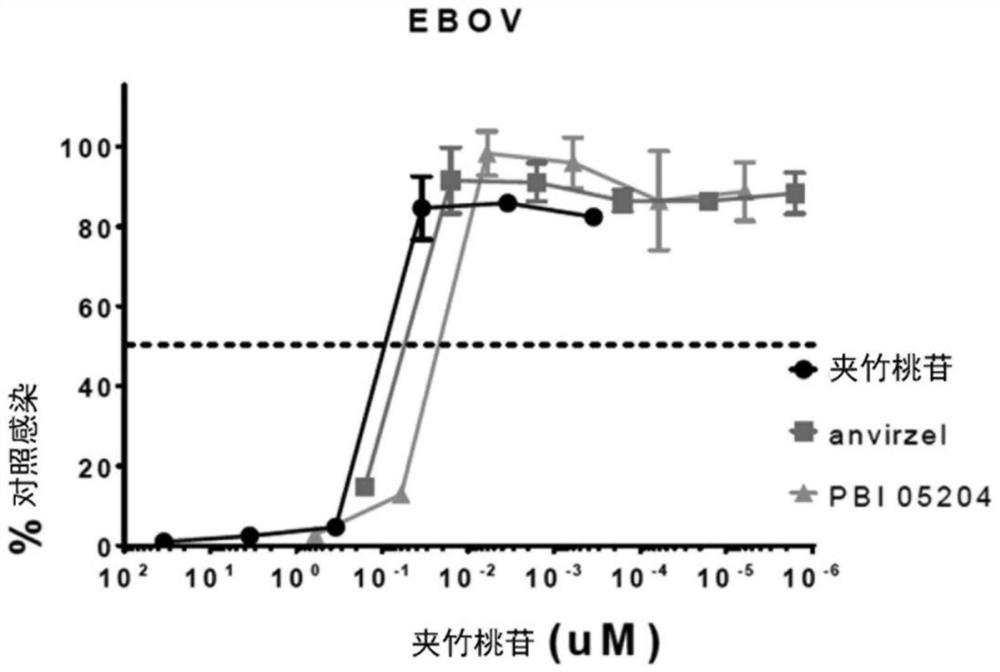
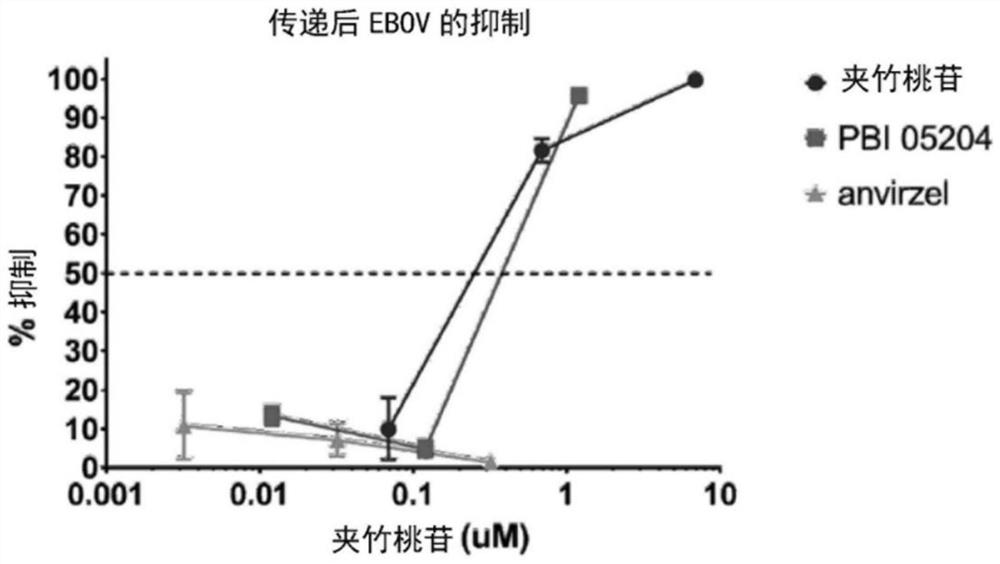
[0130] Example 15 provides a detailed description of the in vitro assay used to evaluate TM (hot water extract of oleander) and PBI-05204 (supercritical fluid (SCF) extract of oleander) against Ebola virus ( Figure 1-2 ) and Marburg virus ( Figure 3-4 ) infection, both Ebola virus and Marburg virus are filoviruses.
[0131] The hot water extract can be administered orally, sublingually, subcutaneously, and intramuscularly. One embodiment is available under the trade name ANVIRZEL TM (Nerium Biotechnology, Inc., San Antonio, TX; Salud Integral Medical Clinic, Tegucigalpa, Honduras; www.saludintegral.com; www.anvirzel.com) is available as a liquid dosage form. For sublingual administration, typical dosing regimens are 1.5 mL per day or three 0.5 mL doses per day. For injection administration, a typical dosing regimen is about 1 to about 2 mL / day, or about 0.1 to about 0.4 ml / m 2 / day for about 1 week to about 6 months or longer, or about 0.4 to about 0.8ml / m 2 / day for ab...
Embodiment 6
[0133]Example 6 provides a detailed description of the in vitro assay used to evaluate the efficacy of cardiac glycosides in the treatment of Zika virus (a flavivirus) infection. In the presence of oleandrin ( Figure 5 ) or digoxin ( Figure 6 ), Vero E6 cells were infected with Zika virus (ZIKV PRVABC59 strain) at an MOI of 0.2. Cells were incubated with virus and cardiac glycosides for 1 hour, after which time the inoculum and unabsorbed cardiac glycosides (if present) were removed. Cells were submerged in fresh medium and incubated for 48 hours before being fixed with formalin and stained for ZIKV infection. The data demonstrate that both cardiac glycosides have antiviral activity against Zika virus; however, oleandrin exhibited a higher (almost 8-fold greater) antiviral activity than digoxin.
Embodiment 14
[0134] Example 14 provides a detailed description of the experiments used to evaluate the antiviral activity of the test compositions against Zika virus and Dengue virus. The data demonstrate that oleandrin shows efficacy against Zika virus and Dengue virus.
[0135] Figure 7 A graph summarizing the in vitro dose-response antiviral activity of various compositions (oleandrin, digoxin, and PBI-05204) against Ebola virus (EBOV) in Vero E6 cells. Figure 8 A graph summarizing the in vitro dose-response antiviral activity of various compositions (oleandrin, digoxin, and PBI-05204) against Marburg virus (MARV) in Vero E6 cells is depicted. Figure 9 To depict a graph summarizing the in vitro cell survival of VeroE6 cells in the presence of various compositions (oleandrin, digoxin and PBI-05204). for Figure 7-8 , exposing the host cell to the composition prior to infection with the virus. EBOV / Kik ( Figure 7 , MOI=1) or MARV / Ci67 ( Figure 8 , MOI=1) to infect Vero E6 cells...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com