A kind of synthetic method of vegfr inhibitor fruquintinib and its benzofuran intermediate
A synthetic method and inhibitor technology, applied in drug combination, organic chemistry, antineoplastic drugs, etc., can solve problems such as unpleasant odor, and achieve the effect of low cost, good reproducibility, and few synthesis steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
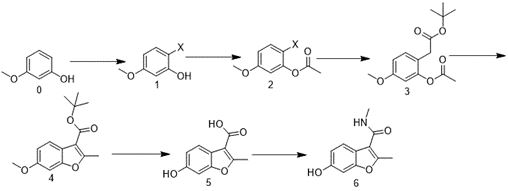
[0074] Embodiment 1 of the present invention is: a synthesis method of a VEGFR inhibitor fruquintinib benzofuran intermediate, comprising the following steps:
[0075] S1, the synthesis of compound 1:
[0076]
[0077] Add 3-methoxyphenol (124.14g) and DMF (250mL) into the reaction flask, control the temperature at 30°C, add NBS (186.88g) in batches, keep warm for 2 hours after the addition, TLC reaction is complete, and the reaction solution Added in water (1000mL), a large amount of solid precipitated out, washed the solid with water, dried to obtain the crude product, crystallized with EA and n-heptane mixed solvent system (the volume ratio of EA and n-heptane was 1:5) to obtain the solid of compound 1 (142.1g, Yield 70.0%, purity greater than 85.0%).
[0078] S2, the synthesis of compound 2:
[0079]
[0080] Add compound 1 (101.5g), TEA (61.2g) and DCM (500mL) into the reaction flask, control the temperature at 30°C, add acetic anhydride (61.2g) dropwise, keep the...
Embodiment 2
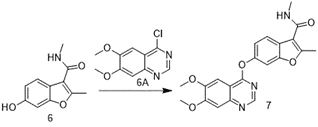
[0093] Embodiment 2 of the present invention is: a method for synthesizing VEGFR inhibitor fruquintinib and intermediates, comprising the following steps:
[0094] S1. Compound 6 was synthesized by the method of Example 1 of the present invention.
[0095] S2, the synthesis of compound 7:
[0096]
[0097] Add compound 6 (10.2g) and acetone (100mL) into the reaction flask, replace with nitrogen, add compound 6A (11.2g), heat up to 60°C for 12 hours after the addition, TLC reaction is complete, cool down to 25°C, add water (300.0g), stirred for 30 minutes, the organic phase was washed once with saturated brine, dried and concentrated to dryness, crystallized with acetone and n-heptane mixed solvent system (the volume ratio of acetone and n-heptane was 2:7) to obtain compound 7 (17.5g , yield 89.0%, product purity greater than 99.0%).
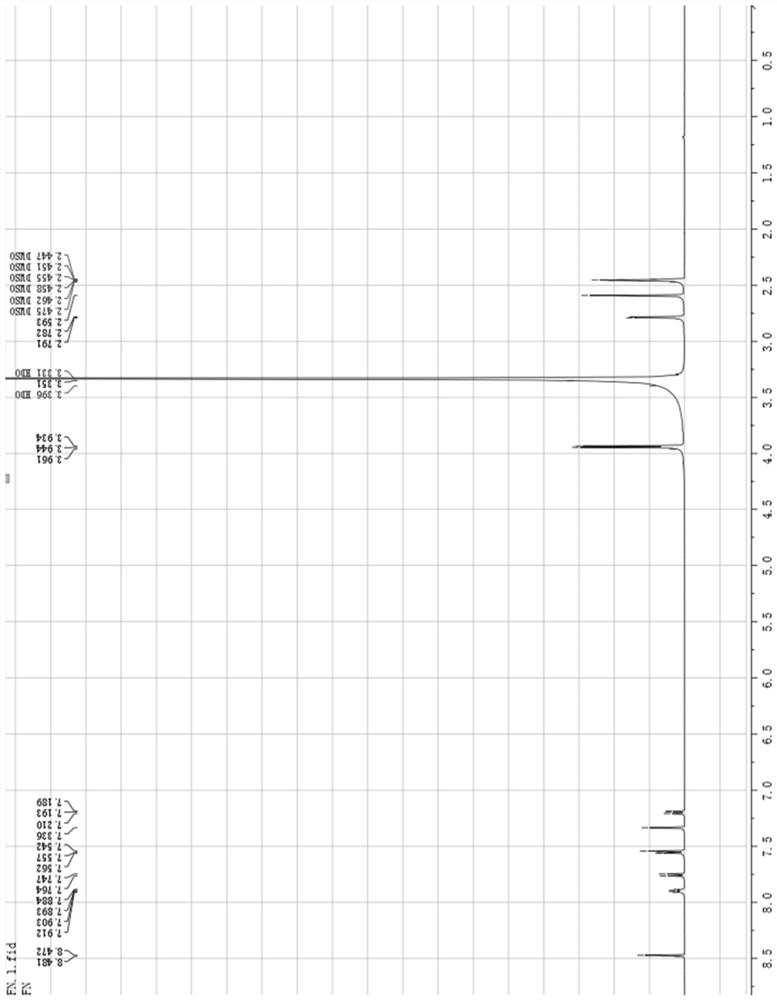
[0098] The nuclear magnetic resonance spectrum of compound 7 prepared by the embodiment of the present invention two is shown in figure 1 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com